Is hclo4 a strong acid
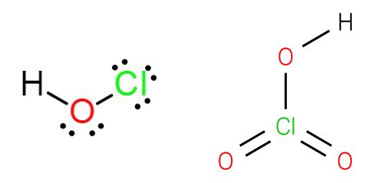
HClO 4 Perchloric acid is an acid. HClO 4 is a proton donor too. Thus, it is classified as an acid. HClO 4 Perchloric acid is a strong acid.
Perchloric acid is a mineral acid with the formula H Cl O 4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate , an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures. Perchloric acid was first synthesized together with potassium perchlorate by Austrian chemist Friedrich von Stadion [ de ] and called "oxygenated chloric acid" in mids. French pharmacist Georges-Simon Serullas introduced the modern designation along with discovering its solid monohydrate which he, however, mistook for an anhydride.
Is hclo4 a strong acid
.
Perchloric acid ydroxidotrioxidochlorine. Acidity p K a. Ca ClO 4 2.
.
Perchloric acid is a mineral acid with the formula H Cl O 4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate , an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures. Perchloric acid was first synthesized together with potassium perchlorate by Austrian chemist Friedrich von Stadion [ de ] and called "oxygenated chloric acid" in mids. French pharmacist Georges-Simon Serullas introduced the modern designation along with discovering its solid monohydrate which he, however, mistook for an anhydride. Perchloric acid is produced industrially by two routes.
Is hclo4 a strong acid
We have seen that the strengths of acids and bases vary over many orders of magnitude. In this section, we explore some of the structural and electronic factors that control the acidity or basicity of a molecule. This effect can be illustrated using the hydrogen halides:. The trend in bond energies is due to a steady decrease in overlap between the 1s orbital of hydrogen and the valence orbital of the halogen atom as the size of the halogen increases. The larger the atom to which H is bonded, the weaker the bond. Thus the bond between H and a large atom in a given family, such as I or Te, is weaker than the bond between H and a smaller atom in the same family, such as F or O. As a result, acid strengths of binary hydrides increase as we go down a column of the periodic table.
Table leg brackets
Cu ClO 4 2. Alsop; Stephen R. GFS chemicals. The crystal structure of HClO4. Solubility in water. Retrieved 24 February March 3, Ba ClO 4 2. Just because a teacher is good at chemistry, this is no indication of their ability to teach you or your child. Germany Israel United States Japan. Dehydration of perchloric acid gives the anhydride dichlorine heptoxide : [9]. Tools Tools. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid , nitric acid and hydrochloric acid. Tm ClO 4 3. Kaiser
Except for their names and formulas, so far we have treated all acids as equals, especially in a chemical reaction.
HClO 4. VV90BG2 Y. Zr ClO 4 4. You will also see its use in chrome etching. Hyperchloric acid [1]. Main hazards. Hf ClO 4 4. EC Number. Ba ClO 4 2. I OClO 3 3. Hafner and H. This form of the acid is stable indefinitely and is commercially available. According to the theories, an acid that completely ionizes or dissociates in water easily is labeled as strong acid.

I do not believe.