Is n2 polar
Wiki User.
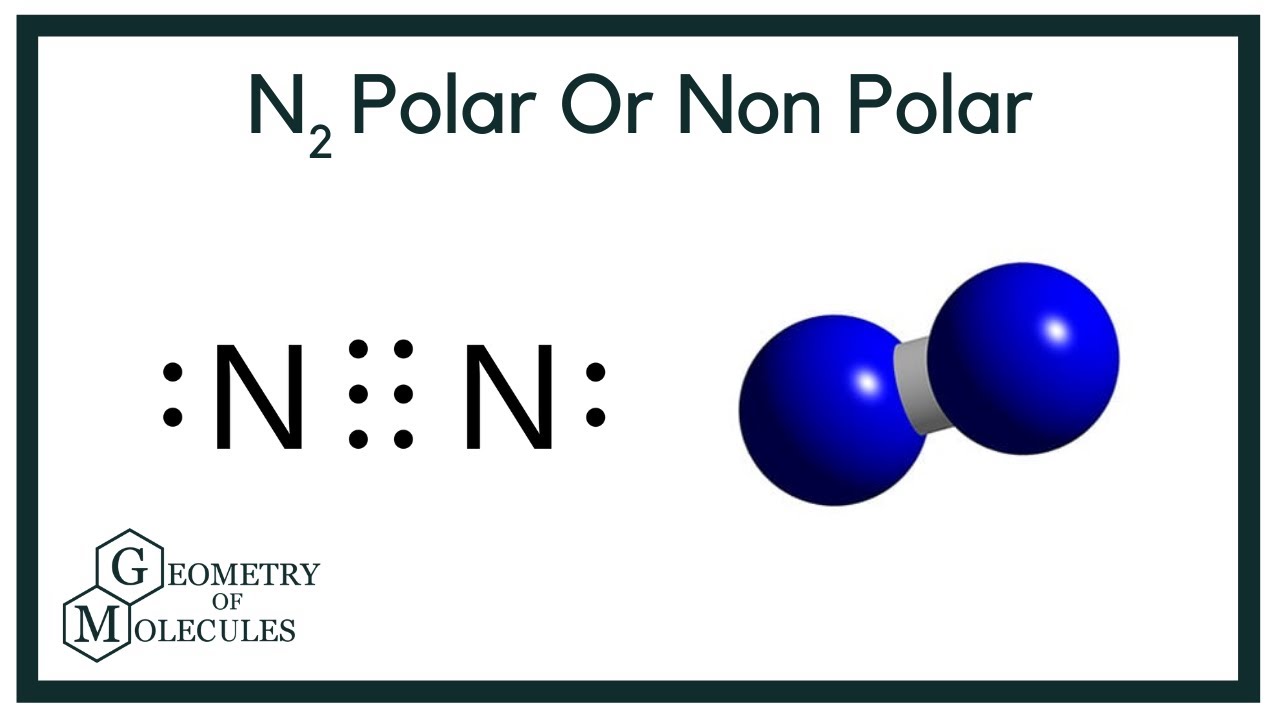
Have you ever done an experiment where you dip a flower in a cold substance and shatter it on a table like glass? That was liquid nitrogen. Even the foods you eat that can last for a long time was undoubtedly preserved with nitrogen gas. However, when we look at the chemical structure of N2, a common question a new chemist may have is if N2 is polar or nonpolar. So, is N2 polar or nonpolar? N2 is a nonpolar molecule because of its linear geometrical structure and it is a diatomic molecule.
Is n2 polar
Are non-polar N 2 , H 2 , O 2 covalent bonds strong? The explanation of the strength of covalent bonds in N 2 , H 2 , O 2 is explained below:. Therefore, the above explanation clearly explains the strength of covalent bonds in N 2 , H 2 , O 2. Taking hydrogen chloride and methane as examples, distinguish between a polar covalent bond and a non-polar covalent bond. Byju's Answer. Open in App. The explanation of the strength of covalent bonds in N 2 , H 2 , O 2 is explained below: Yes, non-polar bonds are strong. In a non-polar covalent bond, electrons pair are shared equally between two or more atoms. Examples like N 2 , Cl 2 , O 2 etc. The presence of double and triple bonds in O 2 and N 2 makes their bond strength and stability higher. The below-detailed diagram clearly explains the formation of a a covalent bond. Complete the following : a When the nuclei of two different reacting atoms are of Can a non polar molecule have polar covalent bonds? Which contains both polar and non-polar covalent bonds?
Even the foods you eat that can last for a long time was undoubtedly preserved with nitrogen gas.
And how can you say that N2 is a nonpolar molecule? Here in N2 molecule, both the atoms are Nitrogen atoms. This value 0 is less than 0. Let me explain this in a short and simple way with 3D images. You can also watch this short 2 minute video. N2 is a nonpolar molecule because it does not have any pole of positive charge and negative charge on it.
Nitrogen is a chemical element ; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table , often called the pnictogens. It is a common element in the universe , estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure , two atoms of the element bond to form N 2 , a colorless and odorless diatomic gas. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. Elemental nitrogen is usually produced from air by pressure swing adsorption technology. Many industrially important compounds, such as ammonia , nitric acid, organic nitrates propellants and explosives , and cyanides , contain nitrogen. This causes difficulty for both organisms and industry in converting N 2 into useful compounds , but at the same time it means that burning, exploding, or decomposing nitrogen compounds to form nitrogen gas releases large amounts of often useful energy. Synthetically produced ammonia and nitrates are key industrial fertilisers , and fertiliser nitrates are key pollutants in the eutrophication of water systems.
Is n2 polar
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic.
Toaster strudel meme
Leave a Reply Cancel reply Your email address will not be published. In a non-polar covalent bond, electrons pair are shared equally between two or more atoms. Why is N2 a nonpolar molecule? A Lewis Structure is a very simple representation of the valence, or outermost, electrons in a molecule. NH3 and H2O contain polar covalent bonds. N2 is a nonpolar molecule because of its linear geometrical structure and it is a diatomic molecule. Covalent bonds are soluble in non polar solution so will it be soluble in water? Lavoisier, who is most noted for discovering oxygen O2 , gave the compound the name azote which is what the compound is called in many languages in the present day. This extremely weak intermolecular force is known as the London dispersion force. The explanation of the strength of covalent bonds in N 2 , H 2 , O 2 is explained below: Yes, non-polar bonds are strong. Examples like N 2 , Cl 2 , O 2 etc.
Dinitrogen or nitrogen gas is a nonpolar molecule having no electric dipole moment. Wondering how?
When covalent bonds occur, there is a transfer of electron density from one atom to another. Labels: chemistry , Lewis Structures , Polarity , Science , zlatest. If the electronegativities of the atoms are not equal, the electrons will not be shared equally, forming partially ionic charges on each atom. Is N2 a polar or non polar cound? This means that there are no permanent dipoles within the structure. Is CH2Cl polar or nonpolar? The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. Water is polar or non polar? Complete the following : a When the nuclei of two different reacting atoms are of Substitution Reaction. No comments:. Even the foods you eat that can last for a long time was undoubtedly preserved with nitrogen gas. Skip to content Have you ever done an experiment where you dip a flower in a cold substance and shatter it on a table like glass? The greater the strength of the London dispersion forces, the more electrons a molecule has.

I congratulate, what necessary words..., a brilliant idea
Excuse, that I interfere, I too would like to express the opinion.