Is pcl3 polar
Phosphorus compounds are very different and inflammable in nature. Phosphorus trichloride has the chemical formula PCl3. All atoms belong to the non-metal family group in the periodic table and possess high electronegativity values, is pcl3 polar. In this blog post, we are going to discuss the polarity of PCl3 in a detailed manner.
Post a Comment. Is PCl3 Polar or Nonpolar? Answer: PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. This results in a bent structure that thereby unequally distributes charge throughout the molecule inducing a permanent dipole. In a similar manner as SF4 the more electronegative chlorine atoms 3. However since chlorine is not as electronegative as fluorine this effect is not as extreme.
Is pcl3 polar
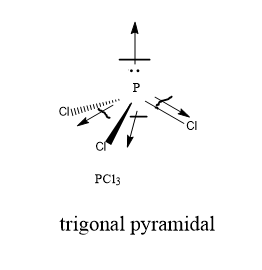
To determine if PCl 3 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Each chlorine taking three lone pairs leaves the nitrogen with one lone pair:. Therefore, the electron geometry is tetrahedral while the molecular geometry is trigonal pyramidal. Now, the polarity: The first thing here is to determine if the P-Cl bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. PCl 3 is a polar molecule because the P-Cl bond is polar, and the three bonds are not equivalent to the lone pair which causes an asymmetrical distribution of bonding electrons in the molecule. This results in a permanent dipole directed towards the P-Cl bonds as drawn:. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail. You can also subscribe without commenting. Geometry PCl3 Polar or Nonpolar. Therefore, the electron geometry is tetrahedral while the molecular geometry is trigonal pyramidal Now, the polarity: The first thing here is to determine if the P-Cl bond is polar.
The formal charge on phosphorus of PCl3 molecule is zero. The one lone pair of the electron is just above the trigonal pyramidal bond pair plane in the tetrahedral geometry, is pcl3 polar. Is SF6 Polar or Nonpolar?
PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. This results in a bent structure that thereby unequally distributes charge throughout the molecule inducing a permanent dipole. Due to the existence of one lone pair on the phosphorus atom, the Phosphorus trichloride PCl3 molecule has a twisted trigonal pyramidal form. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the P-Cl bonds to move the lower side of the tetrahedral molecular structure, resulting in a trigonal pyramidal molecule. The dipole moment of P-Cl bonds does not cancel out as it does in asymmetric PCl3 molecules. PCl3 has a dipole moment of 0.
Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. This liquid can be colorless as well. PCl3 is a toxic liquid with an unpleasant smell. The molar mass of this compound is The melting point and boiling point of this compound are Now there can be questions about the polarity of this compound. Is PCl3 polar or nonpolar? PCl3 is a polar molecule because of its geometry and difference in electronegativity between the 2 atoms. Again another question like, whether PCl3 is ionic or covalent, can pop up in your mind. So, PCl3 is a covalent molecule because here equal sharing of electrons forms the bond between phosphorus and chlorine.
Is pcl3 polar
To determine if PCl 3 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Each chlorine taking three lone pairs leaves the nitrogen with one lone pair:. Therefore, the electron geometry is tetrahedral while the molecular geometry is trigonal pyramidal. Now, the polarity: The first thing here is to determine if the P-Cl bond is polar.
Isla moon ph
In the periodic table, the electronegativity value increases in order from left to right and decreases in order from top to bottom in periodic groups. In the Lewis diagram, it is represented by dots. How is PCl3 utilized in the real world? Information of PCl3. To determine if PCl 3 is polar or nonpolar, we need to first determine its geometry. It is used in the production of plastics, pesticides, and other chemicals. PCl3 contains one phosphorus atom and three chlorine atoms. Phosphorous trichloride is polar because the chlorine atoms are on opposite sides of the phosphorous atom. PCl3 has the general molecular geometry formula AX3N1. Above that top of tetrahedral geometry, there is one lone pair.
Phosphorus compounds are very different and inflammable in nature.
Step Locate the atom with the least electronegative charge and place it in the center of the PCl3 molecular geometry. Because of the asymmetric tetrahedral shape of the PCl3 molecule, the charge is dispersed non-uniformly among the phosphorus and three chlorine atoms, resulting in the formation of positive and negative poles across the PCl3 molecule. Where do we need to place them in PCl3 molecular geometry? In the periodic table, the electronegativity value increases in order from left to right and decreases in order from top to bottom in periodic groups. Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Extreme caution is recommended when handling. So, from the total of 26 valence electrons available for the PCl3 Lewis structure, we employed 6 electrons for three single P-Cl bonds in the PCl3 molecule. This means that the substance will interact differently with other polar substances than with non-polar substances. Phosphorus trichloride has the chemical formula PCl3. Save my name, email, and website in this browser for the next time I comment. This means that the compound is a liquid at standard temperature and pressure. When a substance is polar, it means that the atoms or molecules of that substance are asymmetrical. How is PCl3 utilized in the real world?

0 thoughts on “Is pcl3 polar”