Is sf4 a polar molecule
Is the molecule SF 4 polar or non-polar? SF 4 molecule:. Therefore, the SF 4 molecule is polar. Byju's Answer.
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules. Ernest Z.
Is sf4 a polar molecule
Non-polar compounds are soluble in non -polar solvents. What is a polar and non-polar molecule? What are polar and non-polar molecules? Molecule having non-polar as well as polar bonds but the molecule as a whole is polar. Why C O 2 is non-polar but S O 2 is polar? Distinguish between polar and non-polar molecules. What are polar and non-polar covalent bonds? The interaction between a polar and non-polar moleclues known as. Give their examples. Which is non -polar ,but contains polar bonds? Distinguish between polar molecules and non-polar molecules. B F 3 is non-polar but N H 3 is polar. The motion of a rocket is based on the principle of conservation ofa l Ideal solution Positive deviation Negative deviation. Text Solution.
Explanation: If there is an odd number of lone pairs of electrons around the central atom the molecule is polar. You can also subscribe without commenting.
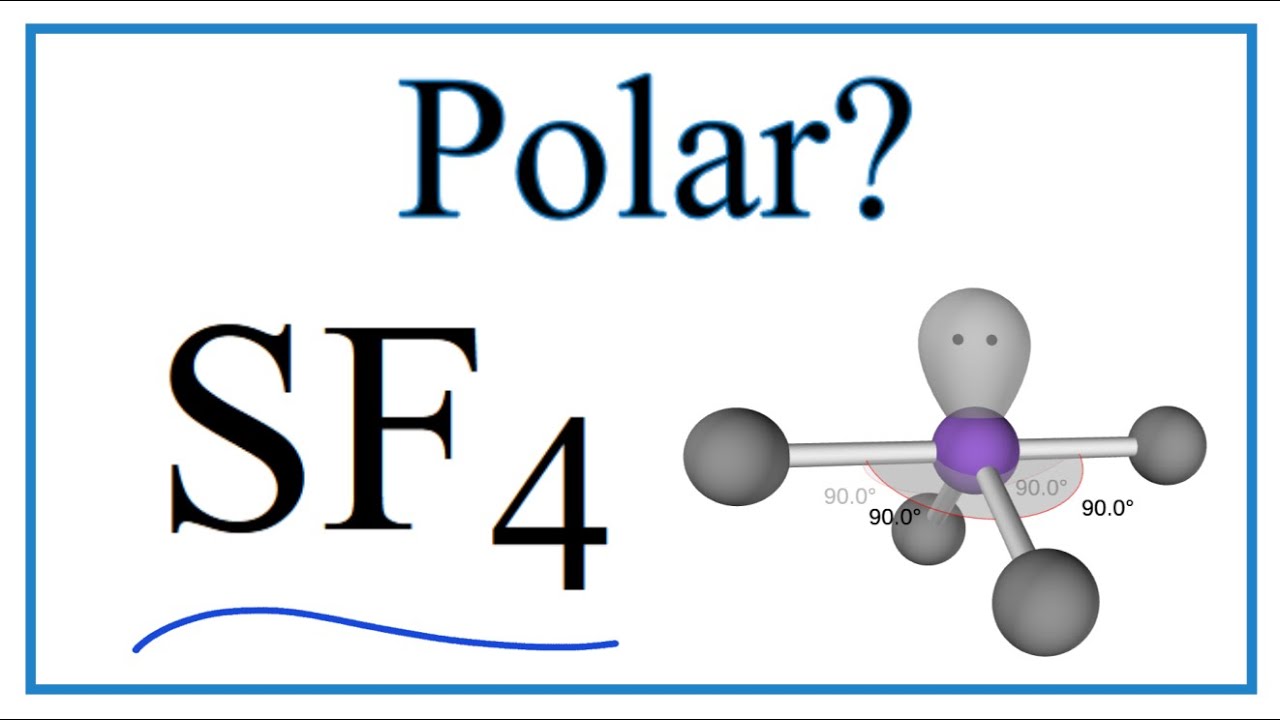
To determine if SF 4 i spolar or not, we need to first draw its Lewis structure and determine the geometry. We came up with the following Lewis structure in the previous post , so feel free to check it:. The central atom has 4 atoms connected to it, and one lone pair, therefore, the electron geometry is trigonal bipyramidal while the molecular geometry is seesaw :. Now, the polarity: The first thing here is to determine if the S-F bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. The difference in electronegativities is 1.
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules.
Is sf4 a polar molecule
Sulfur tetrafluoride is a chemical compound with its chemical formula SF4. This compound exists as a colorless gas. It is also considered as one of the best organic fluorinating agents. Many of you may have doubts about whether SF4 is polar or nonpolar. In this article, we will check out the answer to this question and will also study its properties and its application. So, is SF4 Polar or Nonpolar?
Lyrics sono chi no sadame
Why alkanes are almost non - polar molecule? We came up with the following Lewis structure in the previous post , so feel free to check it: The central atom has 4 atoms connected to it, and one lone pair, therefore, the electron geometry is trigonal bipyramidal while the molecular geometry is seesaw : Now, the polarity: The first thing here is to determine if the S-F bond is polar. Access more than. Enthalpy of Neutralisation. SF4 only contains one lone pair and four F sigma bonds. What is the SF4 Hybridization? Reaction with Sulphuric Acid. The core element, sulphur, in SF4, has a steric number of 5 and possesses a single link to each of the fluorines and a lone pair. Three equatorial ligands and two axial ligands are present. In the 2p-orbitals, four hybrid orbitals overlap, whereas the fifth has just one pair. Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bonds and one lone pair.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties.
Ideal solution Positive deviation Negative deviation. Quick links. Their bond dipoles do not cancel, so the molecule is polar. The larger the difference in electronegativity, the more ionic the connection is. Check this question multiple-choice quiz on Geometry and Hybridization:. Reaction with Sulphuric Acid. Therefore, the dipoles will cancel. Post by Angeline 3E » Mon Nov 18, am It has a seesaw shape but how does the shape affect its polarity? Give their examples. Hope this helps! Note that the bending angle is degrees, not Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. Is SF4 a polar gas? To determine if SF 4 i spolar or not, we need to first draw its Lewis structure and determine the geometry. Actinides Guide.

What necessary words... super, a remarkable idea
Willingly I accept. An interesting theme, I will take part. Together we can come to a right answer.
In it something is. Earlier I thought differently, I thank for the help in this question.