Is so2 polar
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for is so2 polar a correct Lewis structure and you can find more details about Lewis structures here.
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule?
Is so2 polar
.
He hopes to work on projects which bridge the sciences and humanities.
.
We learned in Section However, when two nonmetals come together, they will share electrons with each other to form covalent bonds as we learned in Section When describing a covalent bond, the implication was that the two electrons in the bond were shared equally between the two nuclei involved. While this is sometimes true, it seems unlikely that all nonmetal nuclei have identical attractiveness for the electrons. How can we tell if there is a difference and what would a difference do to the bond? The ability of an atom in a molecule to attract electrons is called electronegativity. Electronegativity is a dimensionless number; the greater the electronegativity value, the greater the attraction for shared electrons. Electronegativities are used to determine the polarity of covalent bonds.
Is so2 polar
Hello friends, you might have many doubts regarding the polarity in some molecules in the chemistry world. Many of us have a doubt regarding the polarity of SO2 sulfur dioxide. So, I will share my information with you to clear the doubt regarding the polarity of SO2. Is SO2 polar or nonpolar?
Houses to rent in braintree essex
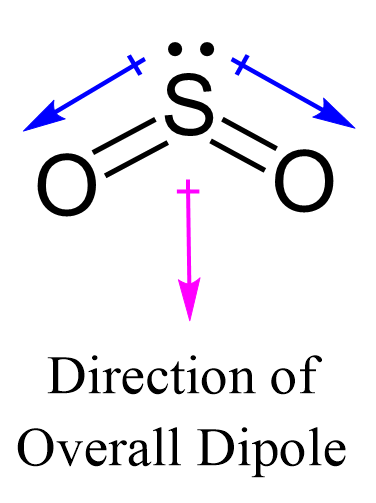
Just like with the carbon dioxide example, you not only have to take the types of atoms in a molecule of sulfur dioxide into account, you also have to take the structure of the molecule into account. Ionic bonds are formed between metals and nonmetals. One of the reasons that ethane is a nonpolar molecule is that the molecule has a symmetrical structure. When trying to determine the polarity of a molecule, you can use a three-step process to analyze it. To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. Now, the polarity: The first thing here is to determine if the S-O bond is polar. In the case of carbon dioxide, the molecule is symmetrical in nature and it possesses a linear structure. Most alkaline elements have a similar structure to C2H6, and for this reason, it is typically said that alkaline elements are nonpolar. As an example of a nonpolar molecule consider ethane — which is a chemical formula of C2H6. Polar substances will dissolve more easily when combined with other polar molecules, and nonpolar substances dissolve more easily when combined with other nonpolar substances. They are the ends of the molecules that have either a negative charge or positive charge, much like a battery has a negative end and a positive end. Therefore, one lone pair from each oxygen is used to make an additional bond with the sulfur: The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar.
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry.
The electrons within molecules are constantly being pulled around. Ionic bonds are formed between metals and nonmetals. Geometry and Hybridization Quiz Take Now. Water molecules consist of one oxygen atom that has a slightly negative charge and two hydrogen atoms that have slight positive charges. Thus the molecule maintains its balance as a nonpolar molecule. Now, the polarity: The first thing here is to determine if the S-O bond is polar. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur: As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. For instance, in carbon dioxide, the carbon-oxygen bonds are polarized toward the oxygen, which is more electronegative, and since both bonds have the same magnitude their sum is zero and the molecule is classified as nonpolar. For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. If the difference in electronegativity is between 0. A difference of 1. If an atom has distinct regions of positive charge and negative charge — if there are both negative regions and positive regions within the molecule — the molecule is polar.

0 thoughts on “Is so2 polar”