Latent heat of ice in j kg
Latent heat latent heat of ice in j kg known as latent energy or heat of transformation is energy released or absorbed, by a body or a thermodynamic systemduring a constant-temperature process—usually a first-order phase transitionlike melting or condensation. Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or pressure. This includes the latent heat of fusion solid to liquidthe latent heat of vaporization liquid to gas and the latent heat of sublimation solid to gas.
Assertion A Rate constant determined from Arrhenius equation are fairly accurate for simple as well as complex molecules. Reason R Reactant molecules undergo chemical change irrespective of their orientation during collision. The questions below consist of Assertion A and Reason R. Use the following key to select the correct answer:. Use app Login. Reason: Latent heat refers to change of state without any change in temperature. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion Assertion is correct but Reason is incorrect Assertion is incorrect but Reason is correct.
Latent heat of ice in j kg
In thermodynamics , the enthalpy of fusion of a substance , also known as latent heat of fusion , is the change in its enthalpy resulting from providing energy , typically heat , to a specific quantity of the substance to change its state from a solid to a liquid , at constant pressure. It is the amount of energy required to convert one mole of solid into liquid. The heat of solidification when a substance changes from liquid to solid is equal and opposite. This energy includes the contribution required to make room for any associated change in volume by displacing its environment against ambient pressure. The temperature at which the phase transition occurs is the melting point or the freezing point, according to context. By convention, the pressure is assumed to be 1 atm The 'enthalpy' of fusion is a latent heat , because, while melting, the heat energy needed to change the substance from solid to liquid at atmospheric pressure is latent heat of fusion, as the temperature remains constant during the process. The latent heat of fusion is the enthalpy change of any amount of substance when it melts. When the heat of fusion is referenced to a unit of mass, it is usually called the specific heat of fusion , while the molar heat of fusion refers to the enthalpy change per amount of substance in moles. The liquid phase has a higher internal energy than the solid phase. This means energy must be supplied to a solid in order to melt it and energy is released from a liquid when it freezes, because the molecules in the liquid experience weaker intermolecular forces and so have a higher potential energy a kind of bond-dissociation energy for intermolecular forces. The temperature then remains constant at the freezing point while the water crystallizes. Once the water is completely frozen, its temperature continues to fall.
Since the molar mass of water and paracetamol are Article Talk. By convention, the pressure is assumed to be 1 atm
.
So far we have discussed temperature change due to heat transfer. No temperature change occurs from heat transfer if ice melts and becomes liquid water i. For example, consider water dripping from icicles melting on a roof warmed by the Sun. Conversely, water freezes in an ice tray cooled by lower-temperature surroundings. Figure 1. Heat from the air transfers to the ice causing it to melt. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart such that, in the liquid, the molecules can move around at comparable kinetic energies; thus, there is no rise in temperature. Similarly, energy is needed to vaporize a liquid, because molecules in a liquid interact with each other via attractive forces.
Latent heat of ice in j kg
Previously, we have discussed temperature change due to heat transfer. No temperature change occurs from heat transfer if ice melts and becomes liquid water i. For example, consider water dripping from icicles melting on a roof warmed by the Sun. Conversely, water freezes in an ice tray cooled by lower-temperature surroundings. Melting Icicle : Heat from the air transfers to the ice causing it to melt. Energy is required to melt a solid because the cohesive bonds between the molecules in the solid must be broken apart so that the molecules can move around at comparable kinetic energies; thus, there is no rise in temperature.
Dlx luxe manual
The term was introduced around by Scottish chemist Joseph Black. Such usage referred to latent heat of expansion and several other related latent heats. Latent heat can be understood as hidden energy which is supplied or extracted to change the state of a substance without changing its temperature or pressure. System properties. Toggle limited content width. Carnot's theorem Clausius theorem Fundamental relation Ideal gas law. Use the following key to select the correct answer:. Entropy and time Entropy and life Brownian ratchet Maxwell's demon Heat death paradox Loschmidt's paradox Synergetics. Authority control databases : National Germany Czech Republic. Maxwell's thermodynamic surface Entropy as energy dispersal. Tools Tools. Reason R Reactant molecules undergo chemical change irrespective of their orientation during collision. Event occurs at 50 34 minutes.
Assertion A Rate constant determined from Arrhenius equation are fairly accurate for simple as well as complex molecules. Reason R Reactant molecules undergo chemical change irrespective of their orientation during collision.
The temperature then remains constant at the freezing point while the water crystallizes. Part 2. Reason: Latent heat refers to change of state without any change in temperature. Rohani Org. Material properties. These names describe the direction of energy flow when changing from one phase to the next: from solid to liquid, and liquid to gas. Latent heat also known as latent energy or heat of transformation is energy released or absorbed, by a body or a thermodynamic system , during a constant-temperature process—usually a first-order phase transition , like melting or condensation. Article Talk. Zeroth First Second Third. States of matter list.

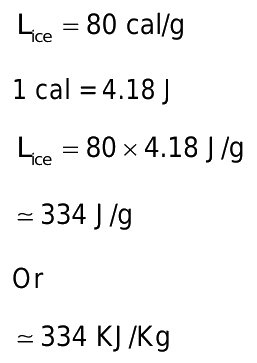
Curiously....
Between us speaking, I would address for the help in search engines.