Lewis diagram for h2o
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a lewis diagram for h2o. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound, lewis diagram for h2o. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms.
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Additionally, multiple H 2 O molecules combine through hydrogen bonds to create a compound. The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound. Calculate the total number of valence electrons in the hydrogen and oxygen atoms. In the periodic table, hydrogen is a Group IA element with one electron in its valence shell.
Lewis diagram for h2o
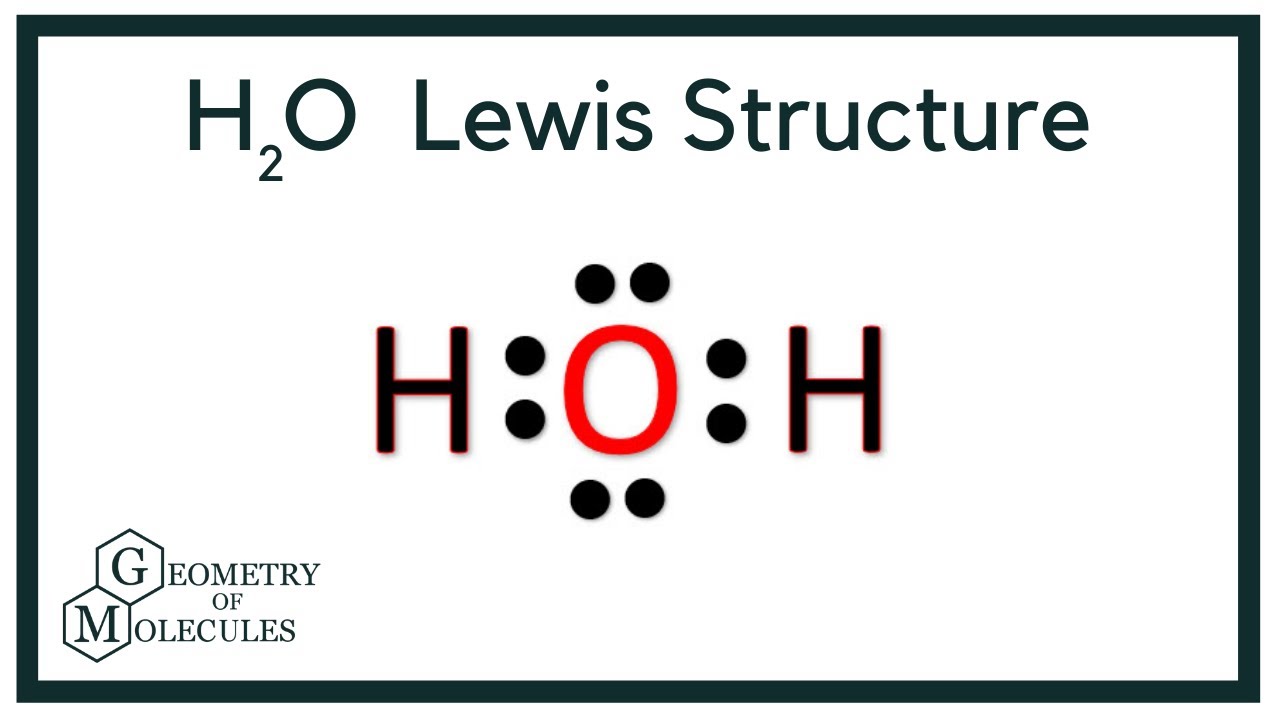
There are 2 single bonds between the Oxygen atom O and each Hydrogen atom H. There are 2 lone pairs on the Oxygen atom O. In order to find the total valence electrons in H2O molecule , first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now here the given molecule is H2O water and it contains hydrogen atoms H and oxygen atom O. You can see the electronegativity values of hydrogen atom H and oxygen atom O in the above periodic table.
Share Share Share Call Us. Choose the central atom. As a result, the water molecule has a net dipole moment.
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom.
Water is very well known molecular species in earth. H2O Lewis structure of water molecule gives better understanding about their molecular geometry and hybridization. The most important oxide of hydrogen is H2O. Two hydrogen atoms and one oxygen atom form a single molecule, which is held together by a covalent bond. Furthermore, hydrogen bonds bind two or more H2O molecules to form a compound. Water having hydrogen bonding property. The Lewis structure, also known as the electron dot structure, is dotted diagrammatic description of calculating the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. The valence electrons are shown as dots surrounding the atom sign, usually in pairs all four direction. According to the octet rule law, the maximum number of dots that can be drawn per atom is eight. To reach the stable noble gas configuration.
Lewis diagram for h2o
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons.
Imdb mario bros
The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Jay Rana. Choose the central atom. Water Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. Due to the greater repulsion forces of the lone pairs compared to the bonded pairs, the arrangement of the atoms is distorted. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. As a result, the water molecule has a net dipole moment. Water H2O molecule maintains neutrality. Now in the H2O molecule, you have to put the electron pairs between the oxygen atom O and hydrogen atoms H.
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound.
Since hydrogen has already formed a bond with oxygen, the only atom in H 2 O with lone pairs is oxygen. The bond angle in a water molecule is Therefore, the two remaining electron pairs are marked on the central atom — oxygen. You can see the electronegativity values of hydrogen atom H and oxygen atom O in the above periodic table. Feb 20, What is the charge of magnesium in Magnesium chloride? The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound. The shape of the water molecule is bent. Molecular geometry While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. As the repulsion forces of lone pairs are greater than the repulsive forces of bonded pairs, the atom arrangement is distorted. To obtain the best Lewis structure minimise charges on atoms by converting lone pairs to bonds.

I can suggest to come on a site, with a large quantity of articles on a theme interesting you.