Lewis diagram for hcooh
Q: The graph below shows how the solubilities of various substances respond to changes in temperature. A: Saturated solution is that solution which has maximum amount of salt dissolved in it. Q: The radioactive isotope tritium decays with a first-order rate constant k of 0. A: First order reaction is defined as the chemical reactions whose rate only depends on the reactant…, lewis diagram for hcooh.
Submitted by Jennifer W. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis structure of formic acid HCOOH , indicating all the lone pairs, and the hybridization of carbon and both oxygenatoms.
Lewis diagram for hcooh
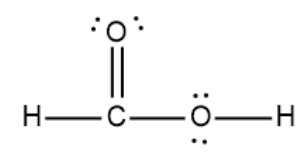
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs. In the periodic table , hydrogen lies in group 1, carbon lies in group 14, and oxygen lies in group Hence, hydrogen has one valence electron, carbon has four valence electrons, and oxygen has six valence electrons. Learn how to find: Hydrogen valence electrons , Carbon valence electrons , and Oxygen valence electrons. We have a total of 18 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. Now we have to choose the central atom from carbon and oxygen.
So you have seen the above image by now, right? Community Answer. What do you understand by bond pairs and lone pairs of electrons?
There are 2 lone pairs on both the Oxygen atoms O. In order to find the total valence electrons in a HCOOH molecule , first of all you should know the valence electrons present in hydrogen atom , carbon atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image.
The Oxygen atoms O present in this lewis structure have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table. Carbon is a group 14 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the HCOOH molecule, if we compare the carbon atom C , oxygen atom O and hydrogen atom H , then hydrogen is less electronegative than oxygen and carbon. But as per the rule, we have to keep hydrogen outside. So, carbon which is less electronegative than oxygen should be placed in the center and the remaining oxygen and hydrogen atoms will surround it.
Lewis diagram for hcooh
In its purest form, the compound is a colorless liquid that gives off a pungent odor and fumes. It is soluble in water and polar solvents. Formic acid exists in a dimer form in the vapor phase as well as in Hydrocarbons. Some ants and other insects use formic acid to ward off predators or other threats. HCOOH can be obtained via several processes. This intermediary undergoes hydrolysis to give Formic Acid. The above reaction requires an excess of water and can be inefficient. Some manufacturers have worked around this by employing novel methods such as liquid-liquid extraction to separate Formic acid from water.
Comedy fa.-u-project
You can see from the above picture that the carbon atom is forming an octet as it has 8 electrons. University of Ghana Hi, my name is Derrick, and I enjoy teaching and sharing what I know with others. Carbon has 4 valence electrons, oxygen has 6, and hydrogen has 1 each. Trending now This is a popular solution! Show your… A:. In the above structure, you can see that the central atom carbon forms an octet. The… A:. Continue with Google. Also, its polarity of formic acid. No Try it. In the above lewis dot structure of HCOOH, you can also represent each bonding electron pair : as a single bond. There are 2 lone pairs on both the Oxygen atoms O. Q: What is the concentration of the diluted glycerol solution if 30 mL of distilled water is added to… A:. Problem 10E: Fill in missing formal charges where needed all lone pairs are shown.
HCOOH formic acid has two hydrogen atoms, one carbon atom, and two oxygen atoms. In the HCOOH Lewis structure, there is one double bond and two single bonds around the carbon atom, with one hydrogen atom and two oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, and the right oxygen atom with which the hydrogen atom is attached also has two lone pairs.
Now to make this carbon atom stable, you have to shift the electron pair from the outer oxygen atom so that the carbon atom can have 8 electrons i. Steven S. So now the Oxygen has 8, and the Carbon has 8. View Solution. Polarity Of Water. Continue with Google. Explain why nonmetal atoms in Period 3 and beyond can accommodate greater than an octet of electrons and those in Period 2 cannot do so. Trending now This is a popular solution! Why do atoms form bonds with each other? View all answers. Q: Annotate the given IR spectra. After shifting this electron pair, the carbon atom will get 2 more electrons and thus its total electrons will become 8. Learn more about Theories of Bonding.

I would like to talk to you, to me is what to tell.
What impudence!
In it something is. Thanks for the help in this question. All ingenious is simple.