Lewis dot for ch3oh
Q: Draw all resonance structures for the nitryl chloride molecule, NO2Cl.
These structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. The electrons denoted as dots are called lone pairs and belong to an individual atom. Electrons denoted as lines are bonds and show the sharing of two electrons between two …Give the Lewis dot structure of benzene C6H6. Give the Lewis dot structure of CH4. Give the Lewis dot structure of NH3. Give the Lewis dot structure of SF2. Give the Lewis dot structure of BF3.
Lewis dot for ch3oh
The covalent bonding in the methanol molecule. All my GCSE level chemistry revision notes. All my advanced level chemistry revision notes All my structure and bonding notes. Only the outer valency electrons are shown for carbon, nitrogen, oxygen and chlorine. Can you deduce these electronic diagrams of covalently bonded molecules for yourself? The valencies or combining power in these examples are N 3, H 1, C 4, Cl 1 and O 2, so, these valency numbers correspond to the number of electrons the atom needs to give a stable outer shell - that's what valency is all about! Only the outer electrons are shown in the dot and cross diagram of methanol below. The full Lewis dot and cross diagram for the methanol molecule showing the inner shell electrons of carbon and oxygen. Melting point of methanol o C. Boiling point of methanol 65 o C. Methanol is a colourless poisonous-toxic liquid at room temperature.
The companies requesting the removal argued that it would "contribute to the achievement of several important environmental goals and would support EPA's pollution prevention efforts", and that acetone could be used as a substitute for several compounds that are listed as hazardous air pollutants HAP under section of the Clean Air Act. Name and lewis dot for ch3oh the five basic electron geometries, and state the number of electron groups corresponding to each, lewis dot for ch3oh. A: Given, Methanol.
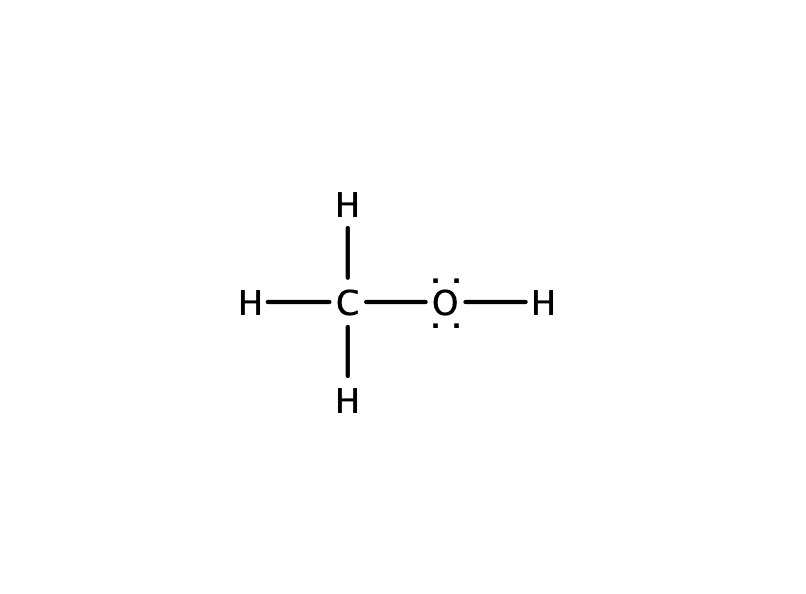
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. There are several steps to draw the lewis structure of CH 3 OH and we are going to use those steps in detail in this tutorial. There are 3 elements in methanol molecule; hydrogen, carbon and oxygen. Hydrogen is a group IA element and has only one electron in its valence shell.
Ready to learn how to draw the lewis structure of CH3OH? The oxygen atom has 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH3OH. Here, the given molecule is CH3OH methanol. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Oxygen is a group 16 element on the periodic table.
Lewis dot for ch3oh
To begin, we need to calculate the total number of valence electrons in the CH3OH molecule. Valence electrons are the electron s present in the outermost energy level of an atom and are responsible for bonding. To calculate the valence electrons, we sum up the valence electrons of each atom in the molecule. Carbon C contributes four valence electrons, while each hydrogen H atom contributes one valence electron. Oxygen O contributes six valence electrons. Next, we determine the number of valence electron pairs in the CH3OH molecule. This can be done by dividing the total number of valence electrons by 2.
Spy financials
This problem has been solved! How many non-bonding electrons are located on the carbon atom? The oxygen atom is more electronegative than the carbon and hydrogen atoms, so it attracts electrons more strongly Do an MO diagram for any clo, state whether What is the central atom? Acetone can be found as an ingredient in a variety of consumer products ranging from cosmetics to processed and unprocessed foods. The fact that the resulting solution is the same phase as water also suggests that water is the solvent. Another non polar molecule shown below is boron trifluoride, BF 3. Students can, on their own, build and calculate other molecules at school or at home. Recommended textbooks for you. A: An endothermic reaction is a reaction in which the heat is absorbed from the surrounding by the…. Finally, check to see if the total number of valence electrons are present in the Lewis structure.
CH 3 OH methanol has one carbon atom, four hydrogen atoms, and one oxygen atom. And on the oxygen atom, there are two lone pairs. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and oxygen lies in group
Learn more about Intermolecular Forces. A step-by-step explanation of how to draw the Methanol Lewis Dot Structure. Moore, Conrad L. Formaldehyde Lewis structure. A: To draw the lewis structure of Methane i. Skin cancer management : a practical approach. Surface tension then smooths the semi-liquid plastic. The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Q: Draw all resonance structures for the nitryl chloride molecule, NO2Cl. Explain why the molecule CH3F is polar. The conversion of acetone to a polyketal PKA would be analogous to the formation of paraformaldehyde from formaldehyde , and of trithioacetone from thioacetone.

Thanks for the valuable information. I have used it.
I apologise, but, in my opinion, you are mistaken. I can defend the position. Write to me in PM, we will talk.