Lewis dot na
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used.
Lewis dot na
The number of electrons in the outermost shell of an atom determines its chemical characteristics. We visualize valence electrons using Lewis dot structures to locate stable electron configurations. In order to attain stability in any atom like noble gases , the majority of atoms often lose or gain electrons. In this article, we will learn how to draw the Lewis dot structure of Sodium Chloride stepwise. A Lewis structure is a type of diagram used to represent the electron configuration of a molecule or ion. It is named after Gilbert Lewis , who first proposed it in The structure consists of a skeletal structure of the molecule, with atoms represented by their chemical symbols and electrons represented by dots. The dots are placed around the chemical symbols to show the distribution of electrons in the molecule. The number of dots around an atom represents the number of electrons that are available for bonding with other atoms in the molecule. The Lewis structure is useful for predicting the geometric shape of a molecule and the chemical properties of its bonds. There can be a maximum of eight dots. This is to ensure that the octet rule may be followed, which only allows for a maximum of eight valence electrons.
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. The dots are placed around the chemical symbols to show the distribution of electrons in the molecule.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1.
Lewis used simple diagrams now called Lewis diagrams to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i. Thus the three atoms shown in Figure 1 from Electrons and Valence can be represented by the following Lewis diagrams:. If the atom is a noble-gas atom, two alternative procedures are possible. Either we can consider the atom to have zero valence electrons or we can regard the outermost filled shell as the valence shell. The first three noble gases can thus be written as:.
Lewis dot na
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Ajith and surya together
Explain why the first two dots in a Lewis electron dot symbol are drawn on the same side of the atomic symbol. Exercises Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Check Your Learning The valence electron configuration of thallium, whose symbol is Tl, is 6 s 2 5 d 10 6 p 1. The central atom of the molecule or ion is chosen because it has the lowest electronegative charge. Questions Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. For oxygen, which has four p electrons, we now have to start doubling up on the dots on one other side of the symbol. For oxygen, which has four p electrons, we now have to start doubling up on the dots on one other side of the symbol. The valence electron configuration for selenium is 4 s 2 4 p 4. A single bond is represented by a line. Draw a Lewis electron dot diagram for an atom or a monatomic ion. Thus the electron dot diagrams for the first column of elements are as follows:. Beryllium has two valence electrons in its 2 s shell, so its electron dot diagram is like that of helium:. Problem What is the Lewis electron dot diagram for each element? The chemical formula of the compound represented by the Lewis dot structure of sodium chloride is NaCl.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
The valence electron configuration for selenium is 4 s 2 4 p 4. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used:. Problem What is the Lewis electron dot diagram for each ion? The structure consists of a skeletal structure of the molecule, with atoms represented by their chemical symbols and electrons represented by dots. The next atom is boron. For example, the electron dot diagram for iron valence shell configuration 4 s 2 3 d 6 is as follows:. Thus we have. There can be a maximum of eight dots. The valence electron configuration for aluminum is 3 s 2 3 p 1. By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we will adopt, although there will be exceptions later. Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol?

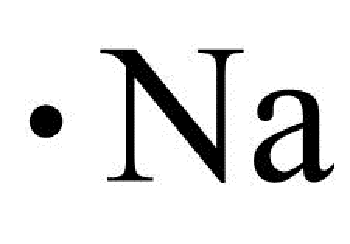
It is a pity, that I can not participate in discussion now. I do not own the necessary information. But with pleasure I will watch this theme.