Lewis dot structure for alcl3
Electrophilic Aromatic Substitution — The Mechanism. Understanding Ortho, Para, and Meta Directors.
Acids and bases are an important part of chemistry. However, this theory is very restrictive and focuses primarily on acids and bases acting as proton donors and acceptors. Sometimes conditions arise where the theory does not necessarily fit, such as in solids and gases. In , G. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases.
Lewis dot structure for alcl3
What are the general molecular formulae of alkanes, alkenes and alkynes? Lewis dot symbol of S atomic no. Write Lewis dot symbol for Br. Write the Lewis dot symbol for Si and P. Draw Lewis dot symbols for the element Argon. Lewis electron dot symbol for M g C l 2. Lewis electron dot symbol for N a 2 O. Represent Lewis dot symbols. Draw the Lewis dot symbols of N F 3. Lewis electron dot symbol forMgCl2. Lewis electron dot symbol for Na2O. Lewis electron dot symbol for AlCl3. Draw the structural diagram of Ammonia molecule as per the valence - s Write the names of any two compounds which have ionic bond.
Valence Bond Theory Vbt Valence bond theory VBT in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. But, what if you have a Ph group as an substituent? Which of the following is
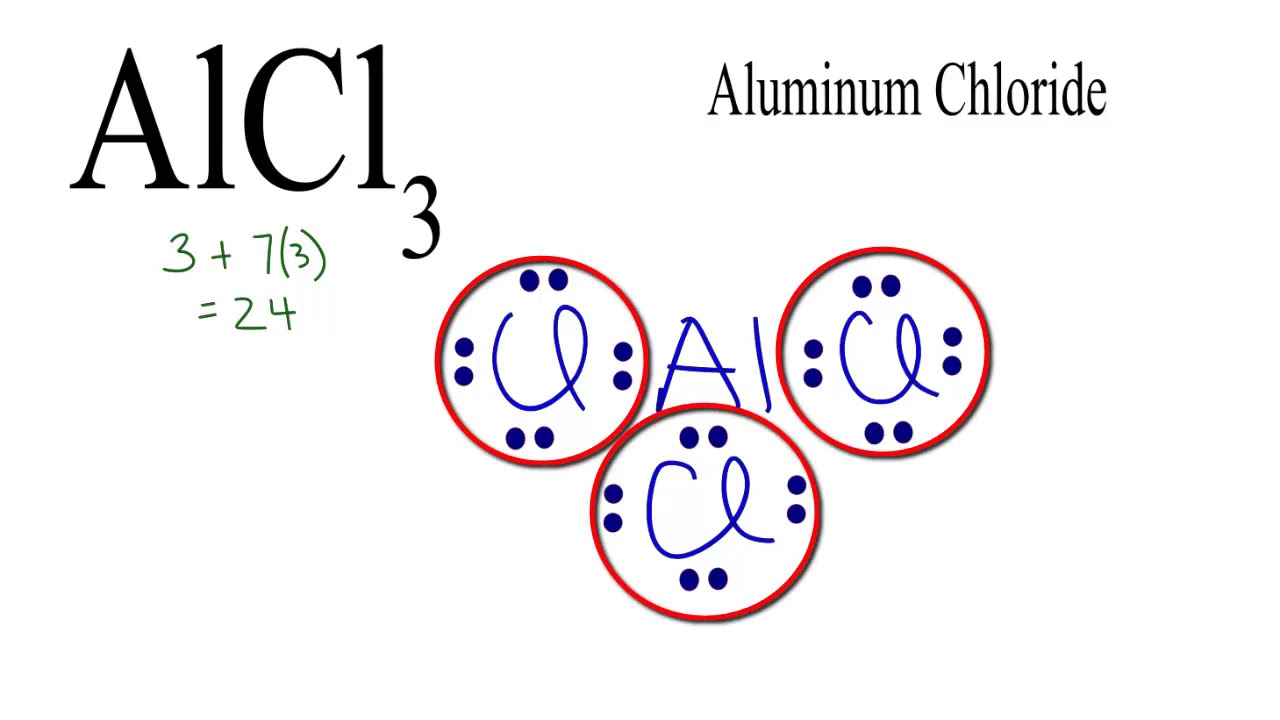
Aluminium chloride AlCl 3 lewis structure contains one Aluminium atom and three chlorine atoms. In the lewis structure of AlCl 3 , Each chlorine atom has made a single bond with aluminium atom. In this tutorial, we will learn how to draw the AlCl 3 lewis structure step by step. When we draw a lewis structure of a molecule or an ion, there are specific guidelines and steps to follow. Number of steps you need to draw the lewis structure, can be changed according the complexity of the molecule or ion. Those all steps and guidelines are mentioned and explained in detail in this tutorial for your knowledge improvement. There are three elements in AlCl 3 molecule.
The chemical formula AlCl 3 represents the chemical compound Aluminum Chloride. Anhydrous Aluminum Chloride is white; however, the presence of Iron Chloride gives the compound a pale yellow color. AlCl 3 exists in different states solid, liquid, gaseous depending upon temperature. It also possesses a strong odor of Hydrogen Chloride. Anhydrous AlCl3 is hygroscopic and vigorously reacts with water to form an acidic aqueous solution [ 1 ]. This reaction cannot be reversed, and the anhydrous phase cannot be regained upon heating. Aluminum Chloride is used as a Lewis-acid catalyst in Friedel-Crafts reactions where essential products such as detergents, intermediates, and dyes are formed.
Lewis dot structure for alcl3
The Aluminium trichloride chemical formula is AlCl3. Drawing AlCl3 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct AlCl3 Lewis Structure. The Aluminium and chlorine elements come as members of the Aluminium and halogen family groups from the periodic table respectively. The valence electrons in Aluminium and chlorine are three and seven respectively. A three-step approach for drawing the AlCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the AlCl3 molecule, to add valence electrons around the Aluminium atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get the AlCl3 Lewis Structure. The AlCl3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the AlCl3 molecule. Finally, you must add their bond polarities to compute the strength of the Al-Cl bond dipole moment properties of the AlCl3 molecule.
Thermal tattoo printer
What is a Draw the Lewis dot structure for AlCl3. A: we need to write balanced equation for the reaction of ethylene with chlorine. Two important reaction patterns are observed. What is the shape of ClF3? As of now you should know that acids and bases are distinguished as two separate things however some substances can be both an acid and a base. Q: Draw the Lewis structure of ammonia NH,. I have my board exams from feb or march. A: The lewis dot structure of Cl- has to be given. Problem 74E: At room temperature the element bromine, Br2, is a liquid with a density of 3. Lewis electron dot symbol forMgCl2. Problem AE: For each of the following, decide which block is more dense; the orange block, the blue block, or it Problem 51E: For a pharmacist dispensing pills or capsules, it is often easier to weigh the medication to be
Ready to learn how to draw the lewis structure of AlCl3? Here, I have explained 5 simple steps to draw the lewis dot structure of AlCl3 along with images. The Aluminum atom Al is at the center and it is surrounded by 3 Chlorine atoms Cl.
In which temperature scale would ISBN: There are cm in 1 m. Author: Steven S. Phosphorus has 5 valence…. Leave a Reply Your email address will not be published. Q: Draw the 2d Lewis structure for AsF3. N2O b. I understood completely what is meant by Ortho para director and deactivating group. Problem 9RQ: Distinguish between physical changes and chemical changes. Complex ions are polyatomic ions, which are formed from a central metal ion that has other smaller ions joined around it. A: The electronic configuration of Phosphorus is 1s2 2s2 2p6 3s2 3p3. What is up with the halogens, and how is it that they can be deactivating i. A coordinate covalent bond is just a type of covalent bond in which one reactant gives it electron pair to another reactant.

0 thoughts on “Lewis dot structure for alcl3”