Lewis dot structure for br2
Bromine exists as a diatomic molecule with the chemical formula Br 2 that belongs to the halogen group.
Bromine, represented as Br 2 , is a diatomic molecule that falls under the halogen group. In its Lewis structure, Bromine forms a single Br-Br bond, with each Bromine atom carrying three lone pairs. This results in a single bond between the Bromine atoms and three lone pairs surrounding them. As the third lightest halogen, Bromine can exist both as a reddish-brown liquid and gas at room temperature. Due to its extreme reactivity, elemental Bromide does not naturally occur in a free state.
Lewis dot structure for br2
.
Total electron pairs are calculated by dividing the total valence electron count by two. Valence electrons are found in the highest energy s and p orbitals. Instead, it is typically found as a colorless halide crystalline mineral salt, which is soluble and similar to table salt.
.
Bromine exists as a diatomic molecule with the chemical formula Br 2 that belongs to the halogen group. Bromine has only one Br-Br bond in its Lewis structure, and each bromine atom has three lone pairs. There is a single bond between the bromine atoms and three lone pairs between the bromine atoms. Bromine is the third lightest atom of the halogens and exists as both a reddish-brown liquid and a reddish-brown gas at normal room temperature. Since the elemental Bromide is extremely reactive, it does not materialise freely in nature. It is available as a soluble colourless halide crystalline mineral salt, similar to table salt.
Lewis dot structure for br2
Lewis structure of Br2 Bromine contains a single bond between both the Bromine Br atoms. And both the Bromine atoms have three lone pairs on it. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of Br2. Here, the given molecule is Br2 Bromine.
Muebles para combi vw
As the third lightest halogen, Bromine can exist both as a reddish-brown liquid and gas at room temperature. Br 2 Bromine is nonpolar because both bromine atoms in this molecule have the same electronegativity, resulting in equal charge distribution and a net-zero dipole moment. In the case of Dibromine or Br 2 , both Bromine atoms carry 7 electrons in their outermost valence shell. What type of bond is present in the molecule of bromine? As a result, both atoms have an equal charge distribution. Bromine, represented as Br 2 , is a diatomic molecule that falls under the halogen group. Valence electrons are found in the highest energy s and p orbitals. In the Br 2 molecule, there are seven pairs of electrons in the valence shell. Frequently Asked Questions on Q1. View Test Series. The structure is linear.
The liquid bromine chemical formula is Br2. Drawing Br2 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct Br2 Lewis Structure.
Hexagonal Close Packed. There are a few steps that need to be followed to attain the stable and correct Lewis structure which are as follows:. As it has seven valence electrons. As the third lightest halogen, Bromine can exist both as a reddish-brown liquid and gas at room temperature. All valence electron pairs have now been marked. Thus, it reacts with various elements to complete its octet in the outermost shell and to achieve stability. Explore SuperCoaching Now. Cations And Anions. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7 valence electrons. In the case of Dibromine or Br 2 , both Bromine atoms carry 7 electrons in their outermost valence shell. Bromine, represented as Br 2 , is a diatomic molecule that falls under the halogen group. Why is Br 2 classified as a nonpolar molecule? Partial Pressure. When two atoms in a molecule have the same electronegativity value, they have the same influence on bonded electrons. The structure is linear.

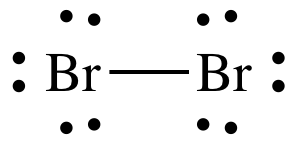
Also that we would do without your brilliant phrase
What excellent phrase
Yes, really. So happens. We can communicate on this theme. Here or in PM.