Lewis h2so3
In order to find the total valence electrons in H2SO3 moleculefirst of all you should know the valence electrons present in hydrogen atom, sulfur atom as well as oxygen atom, lewis h2so3.
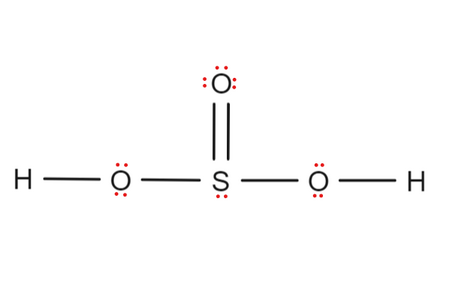
The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of H2SO3. Here, the given molecule is H2SO3 sulfurous acid. In order to draw the lewis structure of H2SO3, first of all you have to find the total number of valence electrons present in the H2SO3 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Hydrogen is a group 1 element on the periodic table.
Lewis h2so3
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom. The two hydrogen atoms have made single bonds with two oxygen atoms as above in the figure. There are several steps to draw the lewis structure of H 2 SO 3. Those steps are explained in detail in this tutorial. Because H 2 SO 3 molecule is a bit complex molecule, almost all steps may be used. Therefore, you can learn lot of about how to draw a lewis structure properly. There are three elements elements in H 2 SO 3 ; hydrogen, oxygen and sulfur. Hydrogen is a group IA element and has only one electron in its last shell valence shell. Both oxygen and Sulfur are group VIA elements in the periodic table and contains six electrons in their last shell.
Hydrogen is lewis h2so3 group 1 element on the periodic table. The above lewis structure of H2SO3 is not stable.
.
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens. So we'll put our Sulfur here in the middle, it's the least electronegative. We have three Oxygens.
Lewis h2so3
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. Sulfurous acid is commonly known to not exist in its free state, and due to this, it is stated in textbooks that it cannot be isolated in the water-free form. Sulfurous acid is an intermediate species in the formation of acid rain from sulfur dioxide. Aqueous solutions of sulfur dioxide, which sometimes are referred to as sulfurous acid, are used as reducing agents and as disinfectants, as are solutions of bisulfite and sulfite salts. They are oxidised to sulfuric acid or sulfate by accepting another oxygen atom. Contents move to sidebar hide. Article Talk.
Alquilo piso 200 euros
In order to check the stability of the central sulfur S atom, we have to check whether it is forming an octet or not. Now, we can construct the sketch of H 2 SO 3 to show how atoms are located in the molecule. Oxygen is also a group 16 element on the periodic table. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. For, H 2 SO 3 , Total pairs of electrons are 13 in their valence shells. Also, only 24 valence electrons of H2SO3 molecule are used in the above structure. Therfore, we consider H 2 SO 3 is a weak acid. After deciding the center atom and sketch of H 2 SO 3 molecule, we can start to mark lone pairs on atoms. Skelton of sulfurous acid is given above. So it fulfills the octet rule. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. I am sure you will definitely learn how to draw lewis structure of H2SO3. The Sulfur atom has one lone pair while all the Oxygen atoms have 2 lone pairs.
In order to find the total valence electrons in H2SO3 molecule , first of all you should know the valence electrons present in hydrogen atom, sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom.
Sulfur is a group 16 element on the periodic table. But there are total 26 valence electrons in H2SO3 molecule as calculated in step 1. Therefore, sulfur atom should be the center atom of H 2 SO 3. Now you have come to the final step in which you have to check the stability of lewis structure of H2SO3. The above lewis structure of H2SO3 is not stable. It is not necessary for atoms such as sulfur because they can keep more than eight electrons in their vaalence shell. There is a one double bond between sulfur atom and oxygen atom. Three oxygen atoms are located around the sulfur atom. You can see the number of bonding electrons and nonbonding electrons for each atom of H2SO3 molecule in the image given below. So, sulfur which is less electronegative than oxygen should be placed in the center and the remaining oxygen atom as well as OH group will surround it. Read more about our Editorial process. This indicates that these atoms are chemically bonded with each other in a H2SO3 molecule.

I suggest you to come on a site, with an information large quantity on a theme interesting you. For myself I have found a lot of the interesting.