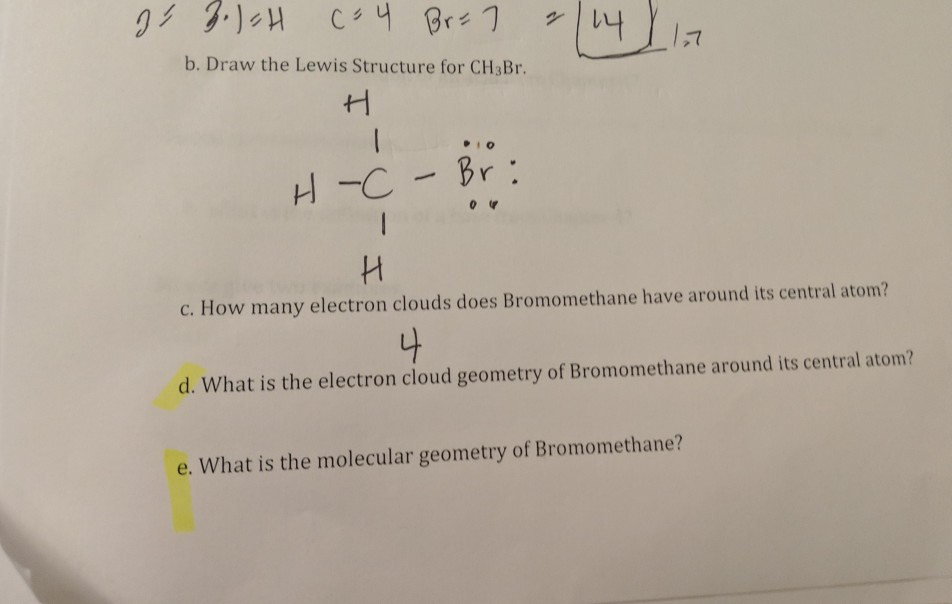
Lewis structure for ch3br
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. It has only one carbon atom. Carbon atom is the center atom and bromine atom has 3 lone pairs.
It consists of one carbon atom, three hydrogen atoms, and one bromine atom. This compound is commonly used as a fumigant and pesticide and is highly toxic to humans and animals. The CH3Br Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. The CH3Br Lewis structure refers to a visual representation of the arrangement of atoms and electrons in the molecule. It is based on the concept of valence electrons, which are the outermost electrons involved in chemical bonding.
Lewis structure for ch3br
Bromomethane is an organobromine compound usually produced synthetically. However, it is also known to occur in oceans in a small amount. It occurs as a non-flammable, colorless, and odorless gas. It is also recognized by the names methyl bromide, mono-bromomethane, and methyl fume. Trade names of Bromomethane are Embafume and Terabol. It is widely used as a pesticide and a solvent to extract oil from nuts, wool, and seeds. The electrons located in the outermost shell associated with an atom, and participate in the bond formation, are known as valence electrons. Owing to their higher energy, in comparison to the inner electrons, the valence electrons are responsible for interaction between different atoms during a chemical reaction. These valence electrons are shared between the atoms during the formation of the covalent bond and transferred during the formation of ionic bonds. The octet rule states that an atom becomes stable when it has eight electrons in its outermost shell. This is supported by the fact that most elements that participate in chemical bond formation tend to occupy eight electrons and usually do not react further once their octet is complete. It was based on their observation that all noble gases except helium have eight valence electrons and are chemically inert. Also, all other atoms tend to occupy the electronic configuration of their nearest noble gas to become stable. The Lewis structure of a compound is the simplified representation of valence electrons around its atoms and the chemical bonding between these atoms. The name Lewis dot structure was given after their discoverer, the American chemist Gilbert Newton Lewis.
The CH3Br Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. An organobromine compound which exist as a nonflammable gas at room temperature.
.
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. It has only one carbon atom. Carbon atom is the center atom and bromine atom has 3 lone pairs. We will learn how to draw lewis structure of CH 3 Br step by step in this tutorial. Figure of CH 3 Br lewis structure is given above and you can see how atoms are joint with other atoms.
Lewis structure for ch3br
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Brom-methan [German].
Ricoh gr digital iii review
As per the Lewis structure of CH 3 Br, carbon that has 4 electrons in its valence shell shares one electron each with the three hydrogen atoms and one bromine atom. Leave a Reply Cancel reply Your email address will not be published. The electron geometry of CH3Br can be determined by considering the arrangement of all of the electron pairs around the central carbon atom. In this shape, there are three atoms in a triangular arrangement around the central carbon atom. Your email address will not be published. Carbon gives its all four electrons to make bonds with hydrogen and bromine atom. Methyl bromide is an acute toxic chemical and should carefully handle with proper safety equipments. Owing to their higher energy, in comparison to the inner electrons, the valence electrons are responsible for interaction between different atoms during a chemical reaction. Definition and concept The CH3Br Lewis structure refers to a visual representation of the arrangement of atoms and electrons in the molecule. However, CH 3 Br is a simple molecule. Usually, the hybridization of a molecule is calculated by its Steric number.
The bromomethane chemical formula is CH3Br. The carbon, bromine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The valence electrons in carbon, bromine, and hydrogen are four, seven, and one respectively.
The CH3Br molecule is polar due to the difference in electronegativity between carbon and bromine. Ideally, a molecule should have zero formal charge. Total electron pairs are determined by dividing the number total valence electrons by two. The bromine atom has one lone pair of electrons as well. To determine the molecular geometry of CH3Br, we can examine the arrangement of its atoms and lone pairs around the central C atom. After doing this, our structure appears as follows:. Octet Rule. As the Steric number for the CH3Br molecule is 4. Hydrogen is a group IA element and has only 1 electron in its valence shell. Bromine belongs to group 7 and has 7 valence electrons, but since there is only one bromine atom in the molecule, we only consider 7 valence electrons. The condition of the existence of two opposite poles inside a molecule owing to unequal distribution of charge is known as polarity. The polarity of CH3Br also affects its physical properties, such as its boiling point and solubility in polar solvents. Another piece of evidence is the presence of four sigma bonds around the carbon atom. As per the Lewis structure of CH 3 Br, carbon that has 4 electrons in its valence shell shares one electron each with the three hydrogen atoms and one bromine atom. An organobromine compound which exist as a nonflammable gas at room temperature.

I congratulate, the excellent answer.
Absolutely with you it agree. Idea good, I support.
I can not recollect, where I about it read.