Lewis structures and vsepr worksheet answers
Open navigation menu.
For complaints, use another form. Study lib. Upload document Create flashcards. Flashcards Collections. Documents Last activity. Add to Add to collection s Add to saved.
Lewis structures and vsepr worksheet answers
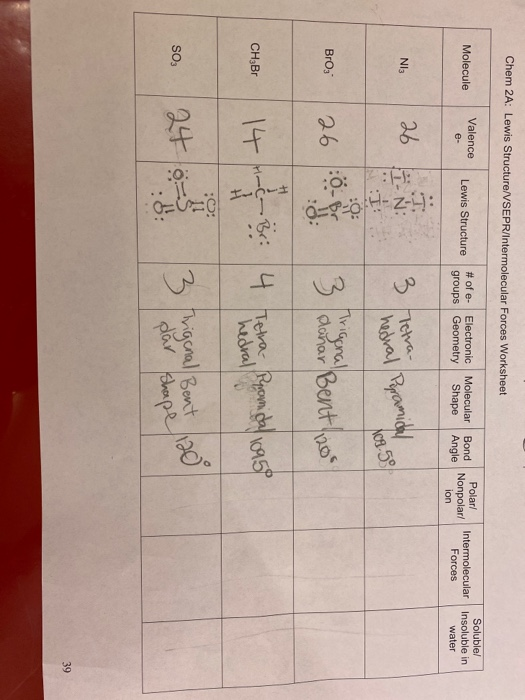
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible. This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom , ignoring all other valence electrons present. According to this model, valence electrons in the Lewis structure form groups , which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron, which in the VSEPR model is counted as a lone pair. Because electrons repel each other electrostatically, the most stable arrangement of electron groups i. In the VSEPR model, the molecule or polyatomic ion is given an AX m E n designation, where A is the central atom, X is a bonded atom, E is a nonbonding valence electron group usually a lone pair of electrons , and m and n are integers. Each group around the central atom is designated as a bonding pair BP or lone nonbonding pair LP. From the BP and LP interactions we can predict both the relative positions of the atoms and the angles between the bonds, called the bond angles.
Chapter 6 Test A Reg Document 4 pages.
Log In Join. View Wish List View Cart. Middle school. High school. Adult education. Resource type. Independent work.
Log In Join. View Wish List View Cart. Middle school. High school. Adult education.
Lewis structures and vsepr worksheet answers
In these practice problems, we will work on determining the electron and molecular geometry of molecules and ions using the VSEPR theory. Determine the electron geometry and molecular geometry of the following molecules using the VSEPR model. For each molecular geometry, determine if there are any lone pairs on the central atom and name the molecular and electron geometries accordingly:. Below is shown the molecular geometry of ReF 7. How would you name this geometry and what bond angles would you expect it to have? For each marked atom, add any missing lone pairs of electrons to determine the steric number, electron and molecular geometry, approximate bond angles, and the hybridization state. You can also download the questions as a PDF worksheet to print and work on here. Notify me of followup comments via e-mail.
Hotwifeexperience chaturbate
NF3 2. Acqmeth Document 6 pages. Adaptive learning for English vocabulary. Word walls. Vsepr Worksheet Document 2 pages. Week 5: Lewis electron. Consequently, molecules with these geometries always have a nonzero dipole moment. Spanish-English dictionary, translator, and learning. There are three nuclei and one lone pair, so the molecular geometry is trigonal pyramidal. To be totally clear, you do NOT need to donate to use all aspects of this site and you never will. Can be used as a bellringer, exit slip, study guide, or quiz. With no lone pair repulsions, we do not expect any bond angles to deviate from the ideal. For complaints, use another form.
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars.
Chemical Bonding Hybridisation Document 7 pages. Unit 1: Introduction: Organic Inorganic Document 1 page. All Resource Types. With five bonding pairs and one lone pair, BrF 5 is designated as AX 5 E; it has a total of six electron pairs. Matching- Types of Bond 6 questions 6. B There are three electron groups around the central atom, two bonding groups and one lone pair of electrons. The oxygens have 2 lone pairs while sulfur had one lone pair. Blog at WordPress. Worksheets, Assessment. Your e-mail Input it if you want to receive answer. Answer key is provided.

0 thoughts on “Lewis structures and vsepr worksheet answers”