Mgcl2 is ionic or covalent
Magnesium chloride is an ionic compound while hydrogen chloride is a covalent compound. But both form ions in their aqueous solutions. Which of the following compounds are ionic and which are covalent?
Since you have started studying chemistry, you are aware of both of these chemical elements called magnesium and chlorine. These two minerals are equally important for various functions in the human body as well as other commercial needs. Magnesium or Mg and chlorine or Cl combine to form magnesium chloride. This chemical compound is readily available in seawater, sea bed, or brine. This mineral can also be extracted by the process of solution mining and evaporation of seawater. Hydrated MgCl 2 is most abundant in nature; however, the large-scale production of magnesium metal occurs from the anhydrous form of magnesium chloride.
Mgcl2 is ionic or covalent
.
All the measurements, as well as temperatures given, are for ml of water. The use of MgCl 2 is also found in de-icing parking lots, sidewalks, and highways in low-temperature areas. Its water solubility for anhydrous is as under:.
.
It has long been known that pure carbon occurs in different forms allotropes including graphite and diamonds. This molecule was named after the architect and inventor R. Buckminster Fuller — , whose signature architectural design was the geodesic dome, characterized by a lattice shell structure supporting a spherical surface. Experimental evidence revealed the formula, C 60 , and then scientists determined how 60 carbon atoms could form one symmetric, stable molecule. They were guided by bonding theory—the topic of this chapter—which explains how individual atoms connect to form more complex structures. As you have learned, ions are atoms or molecules bearing an electrical charge.
Mgcl2 is ionic or covalent
MgCl2 Magnesium chloride is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. Here, Mg is a metal and Cl is a nonmetal. So when they combine, it forms an ionic compound. Well, now you have got to know that MgCl2 is an ionic compound, but let me explain the in-depth reason why MgCl2 is an ionic compound. As mentioned above, you can simply remember that when the metal combines with nonmetal, the bond between them is an ionic bond. Magnesium atom have 12 electrons. Now Magnesium is a metal and the metals are highly electropositive that means they have the tendency to lose electrons and become positive ions. Hence during the chemical reaction, the Magnesium atom will lose 2 electrons to form a stable octet. Chlorine atom have 17 electrons. Now Chlorine is a nonmetal and the nonmetals are highly electronegative that means they have the tendency to gain electrons and become negative ions.
Poweriso review
It is one of the most common reactions of MgCl 2, where the by-product is hydrogen. At the end of this chemical reaction, titanium and magnesium chloride are generated. Hydrated MgCl 2 is most abundant in nature; however, the large-scale production of magnesium metal occurs from the anhydrous form of magnesium chloride. For hexahydrate. Similar Questions. Magnesium or Mg and chlorine or Cl combine to form magnesium chloride. Moreover, by joining our online doubt-clearing classes, you can prepare chemistry at its best level. In this reaction, magnesium chloride and silicon are produced. Thus Magnesium has two electrons in its outermost orbit or it can be said that Magnesium has 2 valence electrons. Also, from brine and Sea, the hydrated Magnesium chloride can be extracted. Magnesium chloride has a crystalline structure. The water solubility of Magnesium Chloride for hexahydrate was grams per ml of water at 20 degrees Celsius.
In chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements.
In its solid state at room temperature, every anion is encircled by cations and vice versa. North America. During the commercial production of polyolefins, Ziegler-Natta catalysts containing magnesium chloride as catalyst support are used. The refractive index of the magnesium chloride I anhydrous is 1. Similar to the human body, plants also need magnesium for their cellular activities. Its boiling point is degrees Celsius, which in Fahrenheit becomes degrees. View Solution. For anhydrous. MgCl 2 suggests magnesium chloride chemical formula in chemistry. Following are the main physical properties of magnesium Chloride. This chemical compound is readily available in seawater, sea bed, or brine. Thus each Chlorine atom has seven electrons in its outermost orbit or it can be said that each Chlorine has 7 valence electrons. From the below content, you can learn the structure, property, preparation, and usages of this compound. Covalent compound Ionic compound Co-ordinate covalent compound Electrovalent compound.

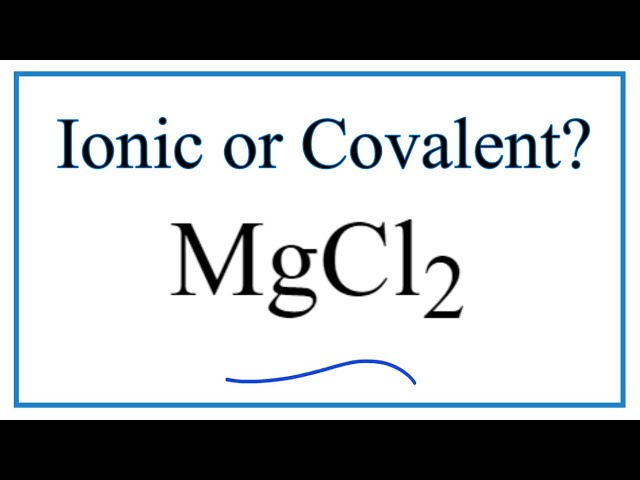
Also what from this follows?
I think, that you are not right. I am assured. Write to me in PM, we will communicate.
I think, that you are not right. I can prove it. Write to me in PM, we will talk.