Molar mass of sodium hydroxide
Molar mass of NaOH Sodium hydroxide is Then, lookup atomic weights for each element in periodic table : Na:
Sodium hydroxide , also known as lye and caustic soda , [1] [2] is an inorganic compound with the formula NaOH. Sodium hydroxide is a highly corrosive base and alkali that decomposes lipids and proteins at ambient temperatures and may cause severe chemical burns. It is highly soluble in water , and readily absorbs moisture and carbon dioxide from the air. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the making of wood pulp and paper , textiles , drinking water , soaps and detergents , and as a drain cleaner. Worldwide production in was approximately 60 million tons, while demand was 51 million tons.
Molar mass of sodium hydroxide
.
Washington Post.
.
You can go from moles of sodium hydroxide, "NaOH" , to grams of sodium hydroxide by using a conversion factor called molar mass. The molar mass tells you the mass of exactly one mole of a given substance. In this case, sodium hydroxide has a molar mass of " You can thus use the molar mass of sodium hydroxide to calculate how many grams you'd get in 0. The answer is rounded to two sig figs , the number of sig figs you have for the number of moles of sodium hydroxide. Stefan V. Jul 27, Explanation: You can go from moles of sodium hydroxide, "NaOH" , to grams of sodium hydroxide by using a conversion factor called molar mass. Related questions How do you calculate the number of moles from volume?
Molar mass of sodium hydroxide
As we described in Section 4. The number of things in a mole is large, very large 6. We are all familiar with common copy-machine paper that comes in sheet reams. If you stacked up 6. The mole is a huge number, and by appreciating this, you can also gain an understanding of how small molecules and atoms really are. Chemists work simultaneously on the level of individual atoms, and on the level of samples large enough to work with in the laboratory. The concept that allows us to bridge these two scales is molar mass. Molar mass is defined as the mass in grams of one mole of a substance. The atomic mass of an element is the relative average of all of the naturally occurring isotopes of that element and atomic mass is the number that appears in the periodic table. We have defined a mole based on the isotopic atomic mass of carbon
Elder kai unlock ability
Nd OH 3. Sodium hydroxide has been used for detection of carbon monoxide poisoning , with blood samples of such patients turning to a vermilion color upon the addition of a few drops of sodium hydroxide. The following colours are observed:. Related: Molecular weights of amino acids. For example, in the petroleum industry, sodium hydroxide is used as an additive in drilling mud to increase alkalinity in bentonite mud systems, to increase the mud viscosity , and to neutralize any acid gas such as hydrogen sulfide and carbon dioxide which may be encountered in the geological formation as drilling progresses. Retrieved July 17, Sodium hydroxide is frequently used in the process of decomposing roadkill dumped in landfills by animal disposal contractors. Chemistry tools. Sodium hydroxide is also widely used in pulping of wood for making paper or regenerated fibers. Yb OH 3. Sodium hydroxide is sometimes used during water purification to raise the pH of water supplies. IDLH Immediate danger. This process was called causticizing.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction.
The viscosity of sodium hydroxide solutions plays a direct role in its application as well as its storage. Enter a chemical formula to calculate its molar mass and elemental composition:. Similar to the hydration of sulfuric acid, dissolution of solid sodium hydroxide in water is a highly exothermic reaction [15] where a large amount of heat is liberated, posing a threat to safety through the possibility of splashing. Related compounds. Sodium hydroxide is used in many industries: in the making of wood pulp and paper , textiles , drinking water , soaps and detergents , and as a drain cleaner. Splashes in the eye can be more serious and can lead to blindness. Chemical compound. Chemistry tools. It is highly soluble in water , and readily absorbs moisture and carbon dioxide from the air. Solid sodium hydroxide is most commonly sold as flakes, prills , and cast blocks. I 4 : 47—

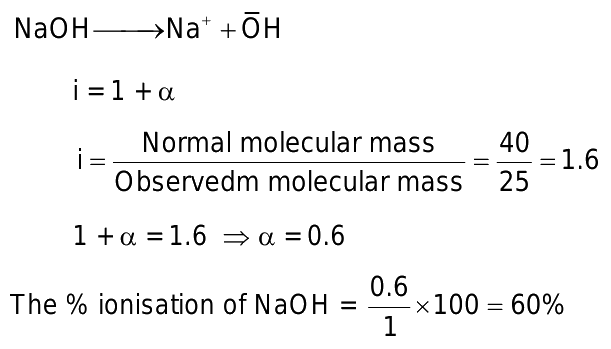
0 thoughts on “Molar mass of sodium hydroxide”