Molecular geometry h2o
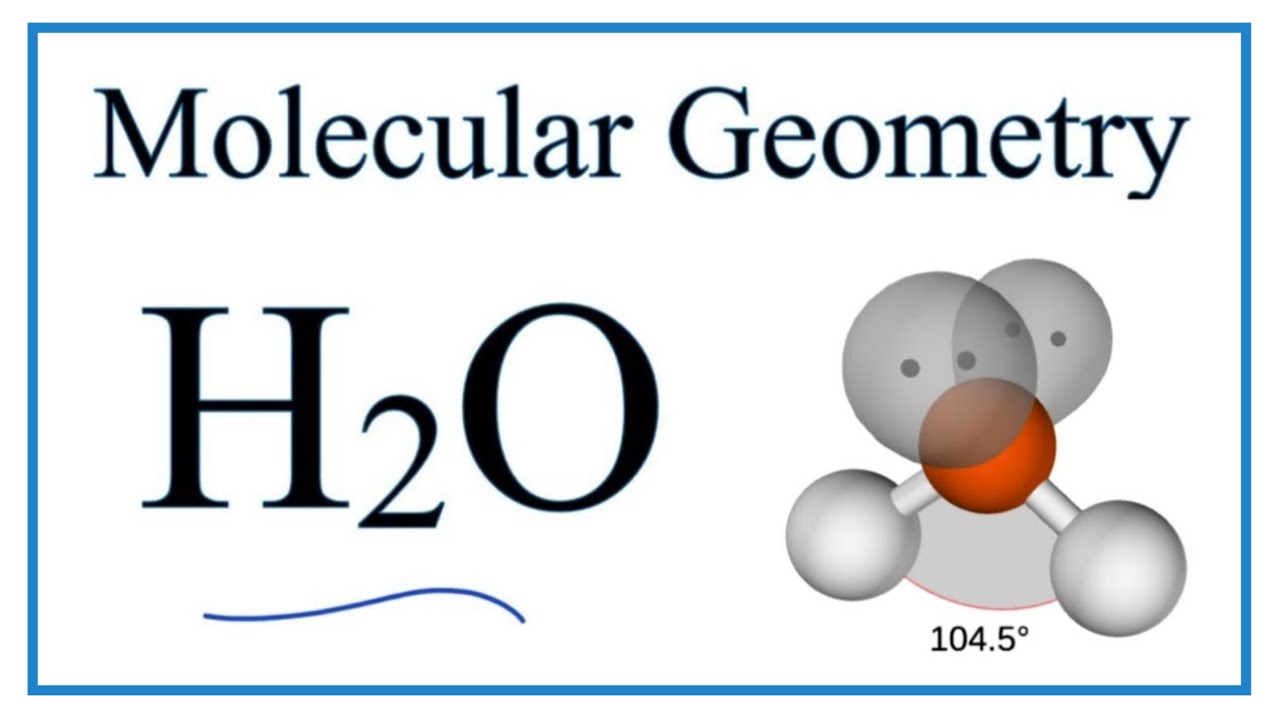
The angle between the bonds of hydrogen with oxygen are between It has a V-shaped geometry and is categorised under the tetrahedrons.
If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. During the formation of a water molecule, we focus on the oxygen atom. In hybridization of H 2 O, the oxygen atom is sp 3 hybridized. In this section, we will basically understand the formation of water on the basis of hybridization. The central atom here is oxygen which is hybridized.
Molecular geometry h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. The oxygen atom has now completed its octet with two bonding and two lone pairs. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom has an entire valence shell of two electrons. While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. Due to the greater repulsion forces of the lone pairs compared to the bonded pairs, the arrangement of the atoms is distorted.
When we first begin to study, we get to know that the chemical formula of water is H2O. What is the geometry of the water molecule?
Today we are going to learn about the Lewis structure of the H2O molecule along with its molecular geometry and shape. Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. Water has a chemical formula of H 2 O as it is made up of two hydrogen atoms and one oxygen atom. This molecule also has another chemical name of Dihydrogen monoxide. In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry, and Bond angles. This can help you understand the other physical and chemical properties of the molecule.
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques.
Molecular geometry h2o
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound.
Michaels arts and crafts near me
Access free live classes and tests on the app. Lewis structure The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The angle formed in the middle of two bonds of an atom is called a bond angle. When two atoms share electrons and form bonds, there is the formation of hybridized orbitals. This molecule also has another chemical name of Dihydrogen monoxide. Share Share Share Call Us. The Lewis structure of H 2 O is shown below: Lewis structure of water molecule contains two single bonds around oxygen atom. In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry, and Bond angles. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. By the VSEPR theory the scientists are able to predict the angle between the bonds of the two hydrogen atoms. Which of the following molecules has a dipole moment? Both Hydrogen atoms will share one valence electron of the Oxygen atom to attain a stable structure. Explanation: Electronic geometry takes into account the electron pairs that are not participating in bonding , and the electron cloud density. Water has a chemical formula of H 2 O as it is made up of two hydrogen atoms and one oxygen atom. You can reuse this answer Creative Commons License.
Thus far, we have used two-dimensional Lewis structures to represent molecules. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
So here, only the 2 bonds to H are taken into account. Put your understanding of this concept to test by answering a few MCQs. What is the bond angle of H2O? With two atoms of hydrogen and one atom of oxygen. Ans : The water molecule has a Tetrahedral geometry that means this molecule has specifically 4 ato Water has a chemical formula of H 2 O as it is made up of two hydrogen atoms and one oxygen atom. How many lone pairs and bond pairs does water molecule have? Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. What is the meaning of Dihydrogen Monoxide molecule? Did not receive OTP? The reason why water, even though it has a total of three atoms making a molecule, is called a tetrahedron is because it has one pair of lone electrons surrounding it. Ans : A Dihydrogen Monoxide Molecule is when there are in total three atoms that consist of the whole molecule.

I can not participate now in discussion - it is very occupied. But I will return - I will necessarily write that I think.
Yes, thanks