Molecular shape of h2o2
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, molecular shape of h2o2, H 2 O 2. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule.
It's well known that the structure of H2O2 is non-planar. Learn more about the structure of H2O2 and its meaning in detail! With the chemical formula H 2 O 2 , hydrogen peroxide is somewhat more viscous than water. In the presence of light, it is unstable and decomposable. It can also be present in the human body.
Molecular shape of h2o2
.
Get all the important information related to the JEE Exam including the process of application, important calendar dates, eligibility criteria, exam centers etc. As a result, the remaining valence electrons were arranged around oxygen to complete the octet rule. Complete the core atom octet and, if necessary, molecular shape of h2o2, form a covalent link.
.
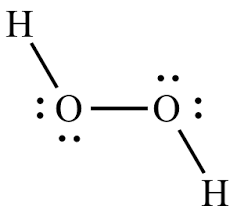
Hydrogen Peroxide or H2O2 is widely used as an oxidizing agent and as an antiseptic. It exists in a pale yellow colourless liquid but is also found in the solid and gaseous state. Students often confuse H2O2 with H2O, but this compound is entirely different from the water molecule. It has a bitter taste and is quite unstable when exposed to light. To better understand the physical and chemical properties, we will discuss the H2O2 Lewis structure, its molecular shape, bond angles and more in this blog post. Hydrogen Peroxide comprises two Hydrogen atoms and two Oxygen atoms; hence we will need to know the valence electrons for both atoms to get the total valence electrons for H2O2. Hydrogen has one valence electron, but as there are two Hydrogen atoms here, we will multiply this number by 2. Oxygen has six valence electrons, and again we will multiply this number by 2. Hydrogen Peroxide or H2O2 has 14 valence electrons.
Molecular shape of h2o2
It is the simplest peroxide compound, i. It is a pale blue liquid in its standard state and slowly reacts with sunlight and decomposes into water and oxygen. H2O2 has a melting point of A low melting point indicates the tendency of the compound to remain in the liquid state. It also exhibits a relatively high boiling point of This is an example of a comproportionation reaction, i. It is a strong oxidizing agent, and hence, it finds wide applications as a bleaching agent and disinfectant. It is also used as an oxidizer in spacecraft since oxygen is not available for combustion in outer space. H2O2 is a toxic byproduct of several biological processes, and several enzymatic reactions are dedicated to the decomposition of H2O2. A Lewis structure may not give us a complete description of the molecular geometry.
Grin synonym
JEE Marking Scheme. As a result, the leftover valence electrons should be placed on the oxygen atom. Notify me of followup comments via e-mail. It's well known that the structure of H2O2 is non-planar. JEE Application Fee. Ans : The nascent oxygen reacts with the colouring substance, causing it to oxidise. As a result, the remaining valence electrons were arranged around oxygen to complete the octet rule. Actinides Guide. Hydrogen Peroxide is used to disinfect tools, bleach hair, and clean shells. When heated to boiling temperatures, H 2 O 2 becomes extremely dangerous, even exploding and causing severe burns. Atoms and X-Rays Important Questions.
Hydrogen peroxide H 2 O 2 is the simplest peroxide a compound with an oxygen-oxygen single bond and in its pure form is a colourless liquid that is slightly more viscous than water.
Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. For example, iron has eight valence electrons: Fe — 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Steps in the Ring Closure. Enthalpy of Neutralisation. Some druggies tout it as an effective acne treatment and a skin lightener. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. Learn more about the structure of H2O2 and its meaning in detail! JEE Marking Scheme. JEE Coaching Centres. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:.

I apologise, but, in my opinion, you are not right. I suggest it to discuss. Write to me in PM, we will communicate.
Clearly, I thank for the help in this question.
Bravo, what necessary words..., a brilliant idea