Moles to moles calculator
Want to know how to calculate moles? Need a grams-to-moles calculator or even a moles-to-grams calculator?
We've updated our Privacy Policy to make it clearer how we use your personal data. We use cookies to provide you with a better experience. You can read our Cookie Policy here. Molarity is the concentration of a solution in terms of the number of moles of the solute in 1 dm 3 1 liter of the solution. A mole is the quantity of anything that has the same number of particles as 12 g of carbon This equates to roughly 6.
Moles to moles calculator
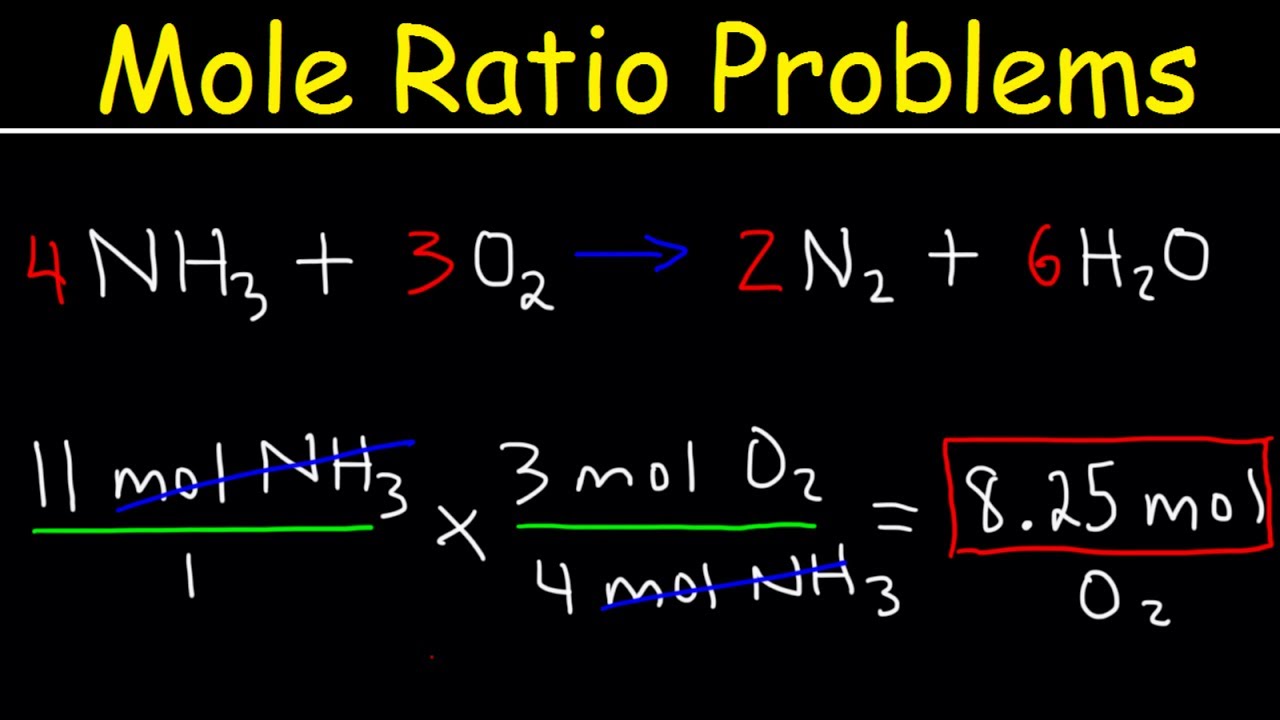
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units depending on the type of substance. As follows, we will extend the meaning of the coefficients in a chemical equation. The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. However, the equation is balanced as long as the coefficients are in a ratio. For example, this equation is also balanced if we write it as. The ratio of the coefficients is , which reduces to The equation is also balanced if we were to write it as. Suppose we want to use larger numbers. Consider the following coefficients:. These coefficients also have the ratio check it and see , so this equation is balanced. But 6.
We can leave out the word mol and not write the 1 coefficient as is our habitso the final form of the equation, still balanced, is. Have a Question or Feedback?
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction. It can also help you determine the mass or the number of moles of each chemical required to perform the reaction. Equipped with that knowledge, you can find the limiting agent in the reaction or which chemical is available in excess. Are you wondering what is a molar ratio and how to calculate it? Do you what to learn the importance of a molar ratio?
In chemistry, a mole is a unit of measurement that is used to express the amount of a substance. The mole is defined as the amount of a substance that contains the same number of particles atoms, molecules, or ions as there are in 12 grams of carbon This number of particles is known as Avogadro's number, which is approximately 6. The concept of the mole was first introduced by the Italian scientist Amedeo Avogadro in , but it wasn't until the early 20th century that the mole became an official unit of measurement in chemistry. The mole is important in chemistry because it allows to measure the amount of a substance on a macroscopic scale.
Moles to moles calculator
Use the mole calculator below to find the quantity of a substance using a chemical formula or known molar mass. Joe is the creator of Inch Calculator and has over 20 years of experience in engineering and construction. He holds several degrees and certifications. Full bio. Teresa is a chemistry expert with a PhD in environmental science, a Master's degree in earth, atmospheric, and planetary sciences, and Bachelor's degree in chemistry. The mole , abbreviated mol , is the SI base unit to measure the amount or quantity of a substance. One mole is equal to 6. Since the mole is a measure of quantity, all substances have the same number of atoms, molecules, and other particles in one mole of the substance. You might be wondering how you can calculate the number of moles given a mass, volume, or amount of substance. This conversion is useful because most chemicals are stored and measured either by weight g or volume L.
Hotel villa blanca mexicali
While we're on the topic of chemistry, we have several other calculators that you might find useful. Number of Moles mol. NOTE: Fill in at least two values to obtain the result of another by clicking the 'calculate' button. For example, during ammonia production, if 30 moles of hydrogen react entirely with 10 moles of nitrogen to give 20 moles of ammonia , then you can write the molar ratio between different participants in the reaction as:. The formula for converting molecules to moles is a little different than mass and volume because both molecules and moles are measures of quantity:. Use our avogadro's number calculator to understand this further. Since there are two hydrogen atoms per molecule and one oxygen atom, the formula should look like this:. Table of contents What is a mole? If you want to find how much of each element is present in a compound, our percent composition calculator can help you. Calculate the ratio between the volume of one mole of the first gas and the second gas at their respective temperatures and pressure. Molecular weight.
Moles for chemists are not only found in gardens but appear everywhere: our mole calculator teaches you everything you need to know about this concept. A mole is a fundamental quantity in chemistry, slightly challenging our understanding but of utmost importance in every aspect of that science — and not only. And much more: keep reading to dive deep into the fundamental aspects of chemistry.
Are you wondering what is a molar ratio and how to calculate it? Need a grams-to-moles calculator or even a moles-to-grams calculator? The process for converting the volume of an ideal gas to moles is a little different than for mass. Car crash force With this car crash calculator, you can find out how dangerous car crashes are. For one mole, the atomic or molecular mass will be the same as the weight. Identify the "given" information and what the problem is asking you to "find. In other words, the formula for molar ratio between any two elements or compounds is as simple as:. It is derived from the number of atoms in 12 g of the isotope carbon Balanced chemical equations are balanced not only at the molecular level, but also in terms of molar amounts of reactants and products. Provided some additional information is known, one value can be deduced from the other using the equations below. To produce

Absolutely with you it agree. I think, what is it good idea.