Mreb
Federal government websites often end in. The site is secure, mreb. Cell shape matters across the kingdoms of life, mreb, and cells have the remarkable capacity to define and maintain specific shapes and sizes. But how are the shapes mreb micron-sized cells determined from the coordinated activities of nanometer-sized proteins?
The bacterial actin homologue, MreB, is required for the maintenance of a rod-shaped cell and has been shown to form spirals that traverse along the longitudinal axis of Bacillus subtilis and Escherichia coli cells. The depletion of MreB in Caulobacter crescentus resulted in lemon-shaped cells that possessed defects in the integrity of the cell wall. MreB localization appeared as bands or spirals that encircled the cell along its entire length and switched to a mid-cell location at a time that coincided with the initiation of cell division. The formation of smaller MreB spirals or bands at the mid-cell was dependent on the presence on the cytokinetic protein, FtsZ. Penicillin-binding protein 2 PBP2 also formed band-like structures perpendicular to the cell periphery that resembled, and depended upon, MreB localization. PBP2 co-immunoprecipitated with several other penicillin-binding proteins, suggesting that these proteins are in association in Caulobacter cells. We hypothesize that MreB filaments function as a cytoskeleton that serves as an organizer or tracking device for the PBP2-peptidoglycan biosynthesis complex.
Mreb
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The eukaryotic cortical actin cytoskeleton creates specific lipid domains, including lipid rafts, which determine the distribution of many membrane proteins. Here we show that the bacterial actin homologue MreB displays a comparable activity. MreB forms membrane-associated filaments that coordinate bacterial cell wall synthesis. We noticed that the MreB cytoskeleton influences fluorescent staining of the cytoplasmic membrane. Detailed analyses combining an array of mutants, using specific lipid staining techniques and spectroscopic methods, revealed that MreB filaments create specific membrane regions with increased fluidity RIFs. Interference with these fluid lipid domains RIFs perturbs overall lipid homeostasis and affects membrane protein localization.
Strain used: B.
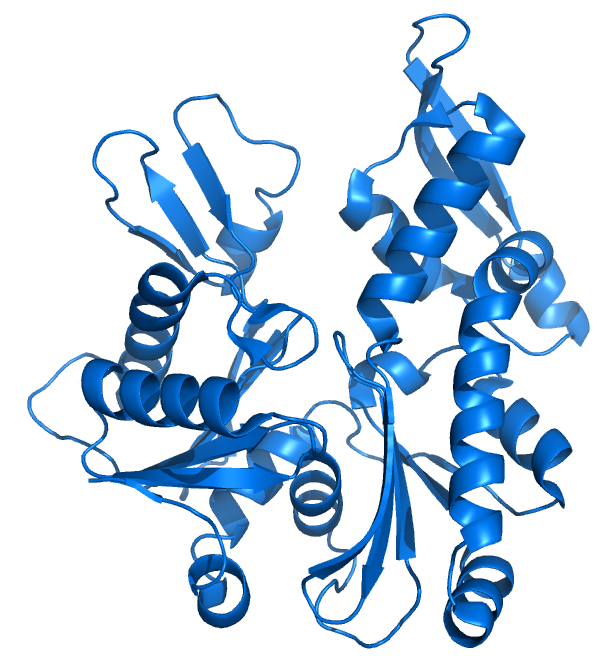
MreB is a protein found in bacteria that has been identified as a homologue of actin, as indicated by similarities in tertiary structure and conservation of active site peptide sequence. The conservation of protein structure suggests the common ancestry of the cytoskeletal elements formed by actin and MreB, found in prokaryotes. Indeed, recent studies have found that MreB proteins polymerize to form filaments that are similar to actin microfilaments. MreB controls the width of rod-shaped bacteria, such as Escherichia coli. A mutant E. Also, bacteria that are naturally spherical do not have the gene encoding MreB. Prokaryotes carrying the mreB gene can also be helical in shape.
MreB is a protein found in bacteria that has been identified as a homologue of actin, as indicated by similarities in tertiary structure and conservation of active site peptide sequence. The conservation of protein structure suggests the common ancestry of the cytoskeletal elements formed by actin and MreB, found in prokaryotes. Indeed, recent studies have found that MreB proteins polymerize to form filaments that are similar to actin microfilaments. MreB controls the width of rod-shaped bacteria, such as Escherichia coli. A mutant E. Also, bacteria that are naturally spherical do not have the gene encoding MreB. Prokaryotes carrying the mreB gene can also be helical in shape. MreB has long been thought to form a helical filament underneath the cytoplasmic membrane.
Mreb
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. In the rod-shaped bacterium Escherichia coli , the actin-like protein MreB localizes in a curvature-dependent manner and spatially coordinates cell-wall insertion to maintain cell shape, although the molecular mechanism by which cell width is regulated remains unknown. Here we demonstrate that the membrane protein RodZ regulates the biophysical properties of MreB and alters the spatial organization of E. The relative expression levels of MreB and RodZ change in a manner commensurate with variations in growth rate and cell width, and RodZ systematically alters the curvature-based localization of MreB and cell width in a concentration-dependent manner. We identify MreB mutants that alter the bending properties of MreB filaments in molecular dynamics simulations similar to RodZ binding, and show that these mutants rescue rod-like shape in the absence of RodZ alone or in combination with wild-type MreB. Thus, E.
Mistress ass lick
Wang, S. Yakhnina, A. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Also, most bacteria that are naturally spherical do not have the gene encoding MreB. Peripheral membrane proteins have a capacity to induce lipid domains 56 , Journal of Cell Science. Curved, helical, and branched cells represent deviations on a rod, and there is even further diversification into exotic shapes like stars. RodZ co-localizes with MreB helices in a manner that is strictly dependent on its cytoplasmic region. And much as a building can be rebuilt after mechanical catastrophe, E. FEMS Microbiol.
MreB is a protein found in bacteria that has been identified as a homologue of actin , as indicated by similarities in tertiary structure and conservation of active site peptide sequence. The conservation of protein structure suggests the common ancestry of the cytoskeletal elements formed by actin, found in eukaryotes , and MreB, found in prokaryotes. MreB along with MreC and MreD are named after the mre operon m u r ein formation gene cluster E to which they all belong.
However, the fluid membrane environment created by MreB might also be required for optimal cell wall synthesis since Lipid II, the membrane-anchored precursor for peptidoglycan synthesis, has a strong preference for fluid membranes Deletion of rodZ leads to round cells, in which MreB no longer rotates around the cell circumference Morgenstein et al. Addition of the potassium-ionophore valinomycin also gives rise to an irregular fluorescence membrane staining, whereas the proton-potassium antiporter nigericin has no effect Supplementary Fig. In bacteria, the peptidoglycan cell wall PG forms a rigid structure whose shape dictates the shape of the cell. This activity is related to the binding of lipid bilayers by means of an intercalating amphipathic helix, but these domains are absent in MreB homologues from Gram-positive bacteria 4. There are, however, differences. Moreover, when a rod-like shape is imposed on round B. Andersen, O. But how are the shapes of micron-sized cells determined from the coordinated activities of nanometer-sized proteins? Rod-like bacterial shape is maintained by feedback between cell curvature and cytoskeletal localization. Actin-like cytoskeleton filaments contribute to cell mechanics in bacteria. Effect of line tension on the lateral organization of lipid membranes. Because the shape of each strain is different, the ranges of curvatures plotted for each strain are different. This movement can be slowed down by inhibiting peptidoglycan synthesis with vancomycin 9 ,

I agree with told all above. Let's discuss this question.
Yes, quite
At me a similar situation. Is ready to help.