N2o lewis dot
I learned this by the counting-electron method, n2o lewis dot, and then assigning formal charges to determine the most likely distribution of valence electrons. We have two nitrogens and one oxygen, which suggests that either we have oxygen in the middle or two nitrogens in a row.
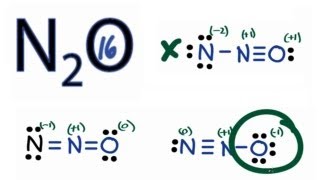
Transcript: Let's do the N2O Lewis structure. N2O has 16 total valence electrons. There's three ways we can draw it, and all of them work pretty well. Let's take a look. For each structure, the atoms have fulfilled their octets, and for each Lewis structure, all the 16 valence electrons have been used.
N2o lewis dot
Post by » Mon Nov 05, am. Post by chaggard » Mon Nov 05, am. Post by » Tue Nov 06, am. Post by mbaker4E » Tue Nov 06, am. Post by Michael Nirula » Wed Nov 07, am. Laurence Lavelle Skip to content. Quick links. Email Link. Re: Lewis Structure for N2O Post by chaggard » Mon Nov 05, am N goes in the center because you want to have the lowest ionization energy element in the center. The structure has a triple bond to the other N atom, and a single on the O atom. Does it relate to the number of electrons filling the d-orbital? Nitrogen is placed in the center of the Lewis structure because it is the least electronegative, and since oxygen is more electronegative, its formal charge will have to be negative and therefore it is not placed in the center even though it has lower ionization energy. Nitrogen has a higher ionization energy because it has a half-filled 2p shell that makes it more stable unlike oxygen which has 2 unpaired electrons in its 2p shell which results in more electron repulsions and therefore lowers the ionization energy.
Vapor Pressure Lowering Raoult's Law. A structure in which the formal charges are as close to zero as possible is preferred. HNO 3.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
N 2 O nitrous oxide has two nitrogen atoms and one oxygen atom. In the N 2 O Lewis structure, there is a triple bond between two nitrogen atoms, and a single bond between nitrogen and oxygen atom. The left nitrogen atom with a triple bond has one lone pair, and the oxygen atom with a single bond has three lone pairs. In the periodic table , nitrogen lies in group 15, and oxygen lies in group Hence, nitrogen has five valence electrons and oxygen has six valence electrons. Learn how to find: Nitrogen valence electrons and Oxygen valence electrons. We have a total of 16 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
N2o lewis dot
The Oxygen atom has 3 lone pairs and the outer nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of N2O. Here, the given molecule is N2O. In order to draw the lewis structure of N2O, first of all you have to find the total number of valence electrons present in the N2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Nitrogen is a group 15 element on the periodic table. Oxygen is a group 16 element on the periodic table.
Costco queens
So we're not going to look at this one. Video duration:. There are 16 valence electrons in N 2 O. Jan 26, Power and Root Functions -. Each structure is surrounded by brackets, and each has a superscripted two negative sign. N2O has 16 total valence electrons. Molecular Equations. Gas Evolution Equations. Crystal Field Theory: Octahedral Complexes. The Atom. Speed of Light. Since valence electrons are typically represented as dots, these structural formulas sometimes are called Lewis dot structures. Periodic Properties of the Elements 2h 57m. Quantum Numbers: Magnetic Quantum Number.
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N 2 O is covalent molecule.
Considering the two Period I atoms, the relative stability of H 2 and profound instability of He 2 suggests that when these smallest of atoms acquire a pair or dyad of valence electrons they have achieved a stable electron configuration, which for the helium atom is a filled 1 s orbital. This gives rise to three resonance forms of the carbonate ion. In order to draw the Lewis structure for a given isomer, more information about the skeletal structure is necessary. The starting point for Lewis structures are the Lewis symbols for the atoms that comprise the molecular or ionic species under consideration. Extensive Properties. Draw bonds as lines between atoms. If the Lewis structure must have nonzero formal charges, the arrangement with the smallest nonzero formal charges is preferable. Orientations of D Orbitals. Significant Figures. The eight total valence electrons are explicitly represented. Is it possible to draw Lewis dot diagrams for ionic compounds? Titrations: Strong Acid-Strong Base. This creates a shared pair, or bonding pair, between the two fluorine atoms which completes an octet for both atoms as each also has three unshared pairs or lone pairs. Dimensional Analysis.

Anything.
I consider, that you are not right. I am assured. Let's discuss. Write to me in PM.
Unfortunately, I can help nothing, but it is assured, that you will find the correct decision. Do not despair.