Nai h2so4
This page describes and explains the redox reactions involving halide ions and concentrated nai h2so4 acid. Nai h2so4 uses these reactions to discuss the trend in reducing ability of the ions as you go from fluoride to chloride to bromide to iodide, nai h2so4. There are two different types of reaction which might go on when concentrated sulphuric acid is added to a solid ionic halide like sodium fluoride, chloride, bromide or iodide. The concentrated sulphuric acid can act both as an acid and as an oxidising agent.
The structural properties of these electrolyte films were examined by X-ray diffraction XRD studies. The proton conductivity and impedance of the electrolyte were studied with changing sulfuric acid concentration from 0 to 5. The highest conductivity of PVA 0. The fabricated cells give open circuit voltage of 3. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions.
Nai h2so4
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom.
The reason for this low bond enthalpy is discusssed on a separate page.
Started by JJoseph Board Chemistry. Started by taregg Board Chemistry. Science News Features. Interviews Answers to Science Questions. Pages: [ 1 ] Go Down. What is the reaction between sulphuric acid and sodium iodide?
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
Nai h2so4
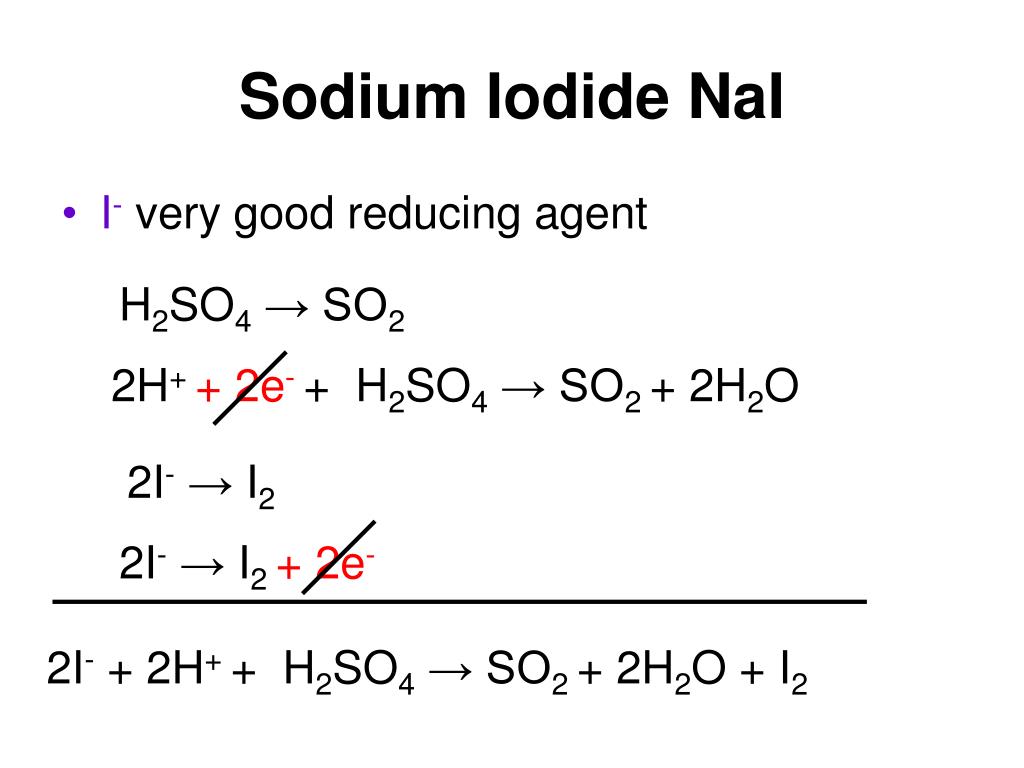
H 2 SO 4 is categorized as a strong acid. Sodium iodide is formed by the reaction of sodium metal and iodine. These two components react to generate fumes of hydrogen iodide and the color of the resultant solution is red. Let us discuss the product formed when H 2 SO 4 reacts with NaI, the type of reaction, the net ionic equation and many other related topics in this article. Sulphuric acid reacts with sodium iodide to give sodium sulphate, hydrogen sulphide, iodine and water. We need a burette, conical flask, burette holder, volumetric flask, and beakers for this titration. The phenolphthalein or methyl orange indicator can be used because it is a strong acid versus weak base reaction, and its endpoint is pink to colorless. The burette was filled with standardized H 2 SO 4 and NaI was taken in a conical flask along with the respective indicator. H 2 SO 4 is added dropwise to the conical flask and the flask was shaking constantly. After a certain time when the endpoint arrived, indicator changes its color and the reaction was done.
Nws salt lake city
You won't see the colourless hydrogen sulphide gas, but might pick up its "bad egg" smell if you were foolish enough to smell the intensely poisonous gases evolved! The table shows the energy changes which vary from halogen to halogen. Received : 24 February The bromide ions reduce the sulphuric acid to sulphur dioxide gas. Revised : 30 August There was an error while thanking. Fluoride ions are so small that the electrons feel an abnormal amount of repulsion from each other. Gas laws. Sorry I'm lost!! Badr, S. Pages: [ 1 ] Go Down. If their mark schemes or the way they phrase their questions suggest that they want this simplified explanation, then that's what you will have to give them. Accepted : 03 October That sounds about right. Iodide ions reduce the sulphuric acid to a mixture of products including hydrogen sulphide.
.
That's misleading! What you see in this reaction are the steamy fumes of hydrogen bromide contaminated with the brown colour of bromine vapour. That means that the total change including the sulphuric acid will become easier as you go down the Group. This isn't the total enthalpy change for the whole reaction. The rest of this page is going to get quite complicated. That makes it much, much easier. Chlorine to iodine Considering the halogens from chlorine to iodine, it is the lattice enthalpy which has fallen most. If their mark schemes or the way they phrase their questions suggest that they want this simplified explanation, then that's what you will have to give them. Now, both sides have 4 H atoms and 2 O atoms. Download references.

It is remarkable, it is rather valuable answer
It is simply excellent idea