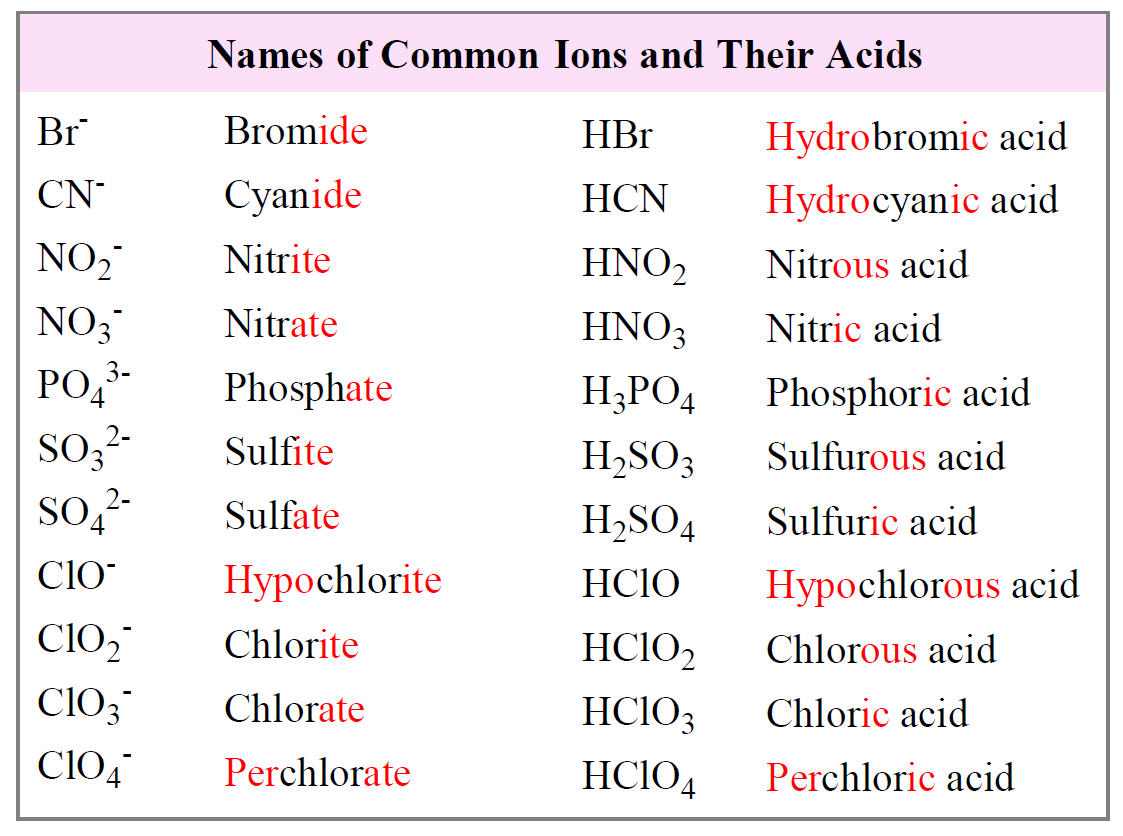
Name of no3
Quantitative metabolomics services for biomarker discovery and validation. Your source for quantitative metabolomics technologies and bioinformatics.
Wiki User. NH4NO3Ammonium nitrate. The name of the compound is silver nitrate. The standard name of this compound is Nitrogen II Oxide. It is generally known as nitric oxide. Pb NO3 4. This compound is beryllium nitrate.
Name of no3
Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. An example of an insoluble nitrate is bismuth oxynitrate. The ion is the conjugate base of nitric acid , consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by resonance structures:. A rich source of inorganic nitrate in the human diets come from leafy green foods, such as spinach and arugula. Drinking water is also a dietary source. Dietary nitrate supplementation delivers positive results when testing endurance exercise performance. Ingestion of large doses of nitrate either in the form of pure sodium nitrate or beetroot juice in young healthy individuals rapidly increases plasma nitrate concentration by a factor of 2 to 3, and this elevated nitrate concentration can be maintained for at least 2 weeks. Increased plasma nitrate stimulates the production of nitric oxide , NO. Nitric oxide is an important physiological signaling molecule that is used in, among other things, regulation of muscle blood flow and mitochondrial respiration.
Clin Chim Acta.
.
Sodium nitrate is the chemical compound with the formula Na N O 3. This alkali metal nitrate salt is also known as Chile saltpeter large deposits of which were historically mined in Chile [4] [5] to distinguish it from ordinary saltpeter, potassium nitrate. The mineral form is also known as nitratine , nitratite or soda niter. Sodium nitrate is a white deliquescent solid very soluble in water. It has been mined extensively for these purposes. The first shipment of saltpeter to Europe arrived in England from Peru in or , right after that country's independence from Spain, but did not find any buyers and was dumped at sea in order to avoid customs toll. In , Ralph Walter Graystone Wyckoff determined its crystal structure using X-ray crystallography. The largest accumulations of naturally occurring sodium nitrate are found in Chile and Peru , where nitrate salts are bound within mineral deposits called caliche ore. For more than a century, the world supply of the compound was mined almost exclusively from the Atacama desert in northern Chile until, at the turn of the 20th century, German chemists Fritz Haber and Carl Bosch developed a process for producing ammonia from the atmosphere on an industrial scale see Haber process. With the onset of World War I , Germany began converting ammonia from this process into a synthetic Chilean saltpeter , which was as practical as the natural compound in production of gunpowder and other munitions.
Name of no3
NO3 is a polyatomic ion with a negative charge. So, it is also referred to by the name of nitrogen oxoanion. The compound has its chemical name as nitrate formed after nitric acid looses a proton from it. Nitrate is an important source of nitrogen and oxygen. It is used as fertilizers like ammonium, sodium, potassium in agricultural farms for higher solubility and biodegradability. It also treats heart pains. NO3 is easily soluble water but too much concentration in drinking water is harmful to human health that affects blood carrying oxygen. In , American chemist, Gilbert N. Lewis introduced the concept of electron dot structure.
Megabus bristol airport
Many meat processors claim their meats e. What is the name for Be NO3 2? Value Source [NO3] -. Quantitative metabolomics services for biomarker discovery and validation. FrNO 3. This condition is called methemoglobinemia and can lead to a lack of oxygen in tissues. J Chromatogr A. RONO 2. Clinical Biochemistry. Hg NO3 2.
Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives.
PMC Isothiocyanate Phosphoramides Sulfenyl chloride Sulfonamide Thiocyanate. For that functional group in medicine, see Nitrovasodilator. Methemoglobinemia can be treated with methylene blue. In particular, nitrate toxicosis in humans occurs through enterohepatic metabolism of nitrates to ammonia, with nitrite being an intermediate. Wiki User. Study now See answers 2. Nitrates lead to the formation of nitrosamines. The sample is introduced with a flow injection analyzer, and the resulting nitrite-containing effluent is then combined with a reagent for colorimetric or electrochemical detection. Retrieved The detection limit is 0.

0 thoughts on “Name of no3”