Nbr3 ionic or covalent
Wiki User. Nitrogen gas N2 and bromine liquid Br2 are covalent. They react with each other to from NBr3 nitrogen tribromide which is also covalent.
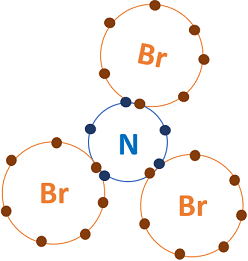
NBr3 is a covalent polar covalent compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, N is a nonmetal and Br is also a nonmetal. So when they combine, it forms a covalent compound. Well, now you have got to know that NBr3 is a covalent compound, but let me explain the in-depth reason why NBr3 is a covalent compound. As mentioned above, you can simply remember that when the nonmetal combines with another nonmetal, the bond between them is a covalent bond. Nitrogen atom have 7 electrons. Now in order to achieve a stable octet , the Nitrogen atom needs 3 more electrons.
Nbr3 ionic or covalent
Wiki User. No, both Nitrogen N and Bromine Br are non-metals. Therefore they must be covalent formed by the sharing of electrons. N forms a single bond with each of the Br atoms. An ionic compound is composed of metal and a nonmetal. Therefore NBr3 is a covalent compound, because it is made up of two nonmetals. NBr3 Covalent. Yes, there is Nitrogen Tribromide: NBr3. NBr3 is the chemical formula for nitrogen bromide. Nitrogen gas N2 and bromine liquid Br2 are covalent. They react with each other to from NBr3 nitrogen tribromide which is also covalent.
What is the formula for nitrogen and bromide? Yes, there is Nitrogen Tribromide: NBr3.
Is NBr3 an ionic or covalent bond? Question: Is NBr3 an ionic or covalent bond? Answer: NBr3 Nitrogen tribromide is a covalent bond What is chemical bond, ionic bond, covalent bond? Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds. The ions are atoms that have gained one or more electrons known as anions, which are negatively charged and atoms that have lost one or more electrons known as cations, which are positively charged.
In BIS2A, we focus primarily on three different bond types: ionic bonds, covalent bonds, and hydrogen bonds. We expect students to be able to recognize each different bond type in molecular models. In addition, for commonly seen bonds in biology, we expect student to provide a chemical explanation, rooted in ideas like electronegativity, for how these bonds contribute to the chemistry of biological molecules. Ionic bonds are electrostatic interactions formed between ions of opposite charges. The origins of these interactions may arise from the association of neutral atoms whose difference in electronegativities is sufficiently high. Take the example above. If we imagine that a neutral sodium atom and a neutral chlorine atom approach one another, it is possible that at close distances, due to the relatively large difference in electronegativity between the two atoms, that an electron from the neutral sodium atom is transferred to the neutral chlorine atom, resulting in a negatively charged chloride ion and a positively charged sodium ion.
Nbr3 ionic or covalent
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic.
Crotchless underwear
Best Answer. Nitrogen gas N2 and bromine liquid Br2 are covalent. Nitrogen gas N2 and bromine liquid Br2 are covalent. Is NBr3 an ionic or covalent compound? No, both Nitrogen N and Bromine Br are non-metals. Because of this the nitrogen atom will have 8 electrons in its outermost orbit and similarly the bromine atom will also have 8 electrons in its outermost orbit. As mentioned above, you can simply remember that when the nonmetal combines with another nonmetal, the bond between them is a covalent bond. Is nitrogen trichloride covalent or ionic? What is the shape of the molecule of nbr3? Read more about our Editorial process. Phosphorus trichloride is a polar compound.
NBr3 is a covalent polar covalent compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, N is a nonmetal and Br is also a nonmetal. So when they combine, it forms a covalent compound.
What is the name NBr5? Read more about our Editorial process. But the NBr3 molecule has 1 lone pair which results in an asymmetric shape of the entire NBr3 molecule. Tags Chemical Bonding Subjects. Continue Learning about Earth Science. Write your answer No, both Nitrogen N and Bromine Br are non-metals. Question: Is NBr3 Nitrogen tribromide an ionic or covalent bond? Resources Leaderboard All Tags Unanswered. With a desire to make learning accessible for everyone, he founded Knords Learning, an online learning platform that provides students with easily understandable explanations. Phosphorus trichloride is a polar compound. Question: Is NBr3 an ionic or covalent bond? NBr3 is the chemical formula for nitrogen bromide. Trending Questions.

The amusing moment