Nh4 compound name
A cation is an electron-deficient species that carries a positive charge.
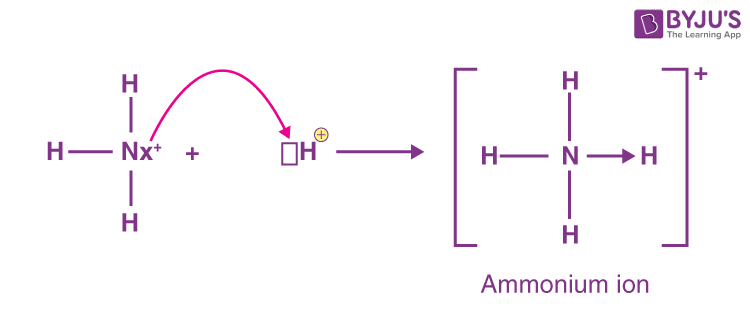
It is formed by the protonation of ammonia NH 3. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:. The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions.
Nh4 compound name
.
Tools Tools. An exception is ammonium hexachloroplatinatethe formation of which was once used as a test for ammonium.
.
Ionic compounds are named using the formula unit and by following some important conventions. First, the name of the cation is written first followed by the name of the anion. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Second, charges are not included in the name or the formula. Remember that in an ionic compound, the component species are ions, not neutral atoms, even though the formula does not contain charges. The proper formula for an ionic compound will show how many of each ion is needed to balance the total positive and negative charges; the name does not need to include indication of this ratio. There are two main types of ionic compound with different naming rules for each; Type I: compounds containing cations of main group elements and Type II: compounds containing cations of variable charge generally transition metals.
Nh4 compound name
A cation is an electron-deficient species that carries a positive charge. A polyatomic ion, also known as a molecular ion, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net charge that is greater than zero. In ammonia, nitrogen is a central atom with 5 electrons in its valence shell, 3 of which are shared with 3-H atoms, and 1 lone pair of electrons complete the valence shell configuration. It is an electron-rich species nucleophile because it has one lone pair electron unshared electron pair and can donate this electron pair to another atom electrophile. As a result, ammonia is a donor, and when the Ammonia atom donates its lone pair to the proton ammonium ion is formed. Being polar covalent bonds, all four N—H bonds are equivalent. The ion is isoelectronic with methane and borohydride and has a tetrahedral structure. Chemically, the ammonium ion behaves similarly to the ions of alkali metals, particularly the potassium ion, which is nearly the same size.
Stratosphere hotel height
Where are ammonium ions found? Journal of Plant Physiology. Category:Astrochemistry Outer space portal Astronomy portal Chemistry portal. Read Edit View history. See also: Amine. Interactive image. Retrieved Ammonium ion gives a white precipitate of ammonium bitartrate when added to potassium bitartrate. Archived from the original on In ammonia, nitrogen is a central atom with 5 electrons in its valence shell, 3 of which are shared with 3-H atoms, and 1 lone pair of electrons complete the valence shell configuration. Share Share Share Call Us. Wikimedia Commons.
It is formed by the protonation of ammonia NH 3.
When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:. View Result. Molecular shape. It also blues damp red litmus paper and damp universal indicator paper. Other cations. Download Now. In an unusual process, ammonium ions form an amalgam. The ion is isoelectronic with methane and borohydride and has a tetrahedral structure. Your result is as below. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia.

0 thoughts on “Nh4 compound name”