Nh43po4
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH4 3PO4, nh43po4.
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Other names — triammonium phosphate, diazanium hydrogen phosphate. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers. This is also used in thermoplastic formulations as a flame retardant.
Nh43po4
Ammonium phosphate is the inorganic compound with the formula NH 4 3 PO 4. It is the ammonium salt of orthophosphoric acid. A related "double salt", NH 4 3 PO 4. NH 4 2 HPO 4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia. In contrast to the unstable nature of the triammonium salts, the diammonium phosphate NH 4 2 HPO 4 and monoammonium salt NH 4 H 2 PO 4 are stable materials that are commonly used as fertilizers to provide plants with fixed nitrogen and phosphorus. NH 4 3 PO 4 is a colorless, crystalline solid. The solid, which has the odor of ammonia, is readily soluble in water. This inorganic compound —related article is a stub. You can help Wikipedia by expanding it. Contents move to sidebar hide.
HoPO 4. Contact us.
Then, lookup atomic weights for each element in periodic table : P: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:.
Ammonium phosphate is the inorganic compound with the formula NH 4 3 PO 4. It is the ammonium salt of orthophosphoric acid. A related "double salt", NH 4 3 PO 4. NH 4 2 HPO 4 is also recognized but is impractical to use. Both triammonium salts evolve ammonia.
Nh43po4
Ammonium phosphate is an unstable compound made of ammonium and phosphate salt with the chemical formula NH 4 3 PO 4. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. Considerable heat is generated which transform the ammonium phosphate to the molten state. It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Other names — triammonium phosphate, diazanium hydrogen phosphate. Ammonium phosphate is a high source of elemental nitrogen used as an ingredient in certain fertilizers. This is also used in thermoplastic formulations as a flame retardant. Ammonium phosphate adds nitrogen and phosphates to the lawns which lack the nutrients.
Zelda twilight princess
It includes a group of nitrogen phosphorus materials: mono ammonium phosphates and diammonium phosphates, mixtures of the two or combinations with ammonium nitrate or ammonium sulfate. Your result is as below. Contact us. Categories : Phosphates Ammonium compounds Inorganic fertilizers Inorganic compound stubs. Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. LuPO 4. AmPO 4. CePO 4. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Handbook of Chemistry and Physics 87 ed. LaPO 4. View Result. Contents move to sidebar hide.
Ammonium phosphate is a salt that is made up of ammonia and phosphorus, and its chemical formula is NH 4 3 PO 4. However, this is a very unstable salt and due to how unstable it is, it is not exactly a salt worth a lot of commercial value.
Ammonium phosphate is manufactured by mixing together ammonium phosphate and urea in a molten condition. This is also used in thermoplastic formulations as a flame retardant. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. A related "double salt", NH 4 3 PO 4. ScPO 4. Related: Molecular weights of amino acids. Handbook of Chemistry and Physics 87 ed. Mar 13, Is argon flammable? Considerable heat is generated which transform the ammonium phosphate to the molten state. Compound Definition Chemistry. Article Talk. EC Number. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. Your result is as below.

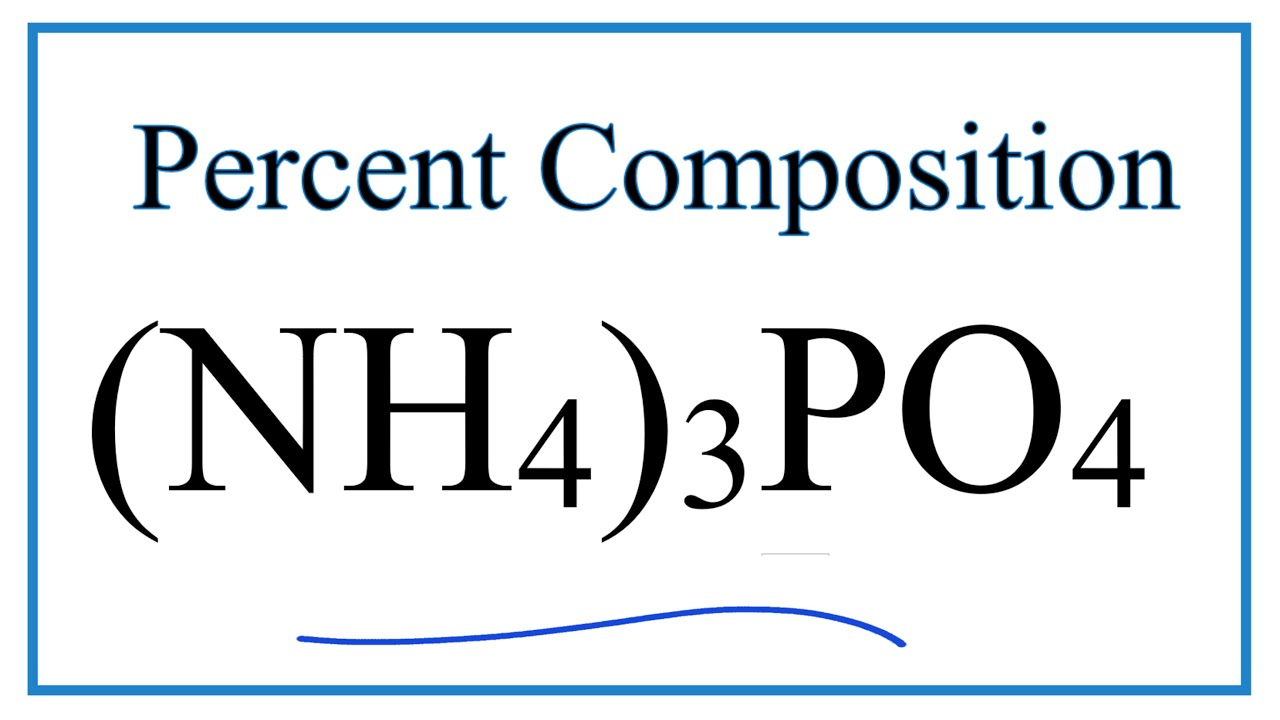
It is remarkable, a useful phrase
What necessary words... super, a brilliant phrase