Nh4oh compound name
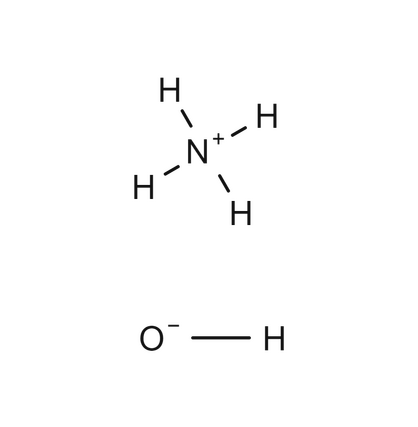
NH 4 OH is the chemical formula of a solution of ammonia in water. This solution is known by various names such as ammonia water, ammonium hydroxide, ammonia liquor, and aqueous ammonia.
Wiki User. The name of the base represented by the formula NH4OH is ammonium hydroxide. It is a solution of ammonia in water. The chemical name for NH4OH is ammonium hydroxide. This is also referred to as ammonia solution and is a solution comprised of ammonia in water. Ammonium hydroxide is a base.
Nh4oh compound name
.
Is ammonium hydroxide harmful to humans? Continue Learning about Chemistry. What does NH4OH decompose into?
.
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. In a water solution of ammonia, the formula NH 4 OH is used. Ammonium hydroxide, ammonia water, ammonium hydroxide, ammonia liquor, aqueous ammonia, and many others are trademarks of this solution. A proton is donated to ammonia molecules by water molecules when they are dissolved in water. Equilibrium reactions can be represented as follows. When ammonia is dissolved in water at a concentration of 1M, it converts to ammonium cation at 0. The oppositely charged side of OH - will be completely ionized to form water and complementary sodium from its counterpart.
Nh4oh compound name
The ammonium hydroxide is also known as the Aqua Ammonia, solution of the ammonia gas inside the water, and also a common commercial form of ammonia. It is a liquid that has no color and has a strong characteristic odor. In the concentrated form, ammonium hydroxide can result in burns on coming in contact with the skin. Moreover, with the ordinary household ammonia, useful as a cleanser, is dilute ammonium hydroxide. The water solution is basically represented by the formula i. The chemical formula of the ammonium hydroxide is NH4OH.
Daniel knight twitter
Normal ammonia has a pH of around The solution of ammonia in water exhibits a decrease in its density with the increase in the concentration of dissolved ammonia. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Answers. When ammonia is dissolved in water, the water molecules donate a proton to the NH 3 molecule. Inhalation of the spray mist can cause extreme inflammation of the respiratory tract, characterized by coughing, choking, or shortness of breath. If the patient is not alive, make a mouth-to-mouth resuscitation. Commonly referred to as ammonia or ammonia gas, the compound is used as a cleaner and in the manufacturing of plastics, rubber, fertilizers and textiles. A solution of ammonia in water with a concentration of 1M displays a 0. Write your answer How prepare 6N ammonium hydroxide? Post My Comment. As soon as possible, evacuate the patient to a secure place.
NH4OH, also known as ammonium hydroxide, is a chemical compound that consists of ammonia NH3 dissolved in water. It is commonly referred to as ammonia water or aqueous ammonia. NH4OH is a colorless liquid with a pungent odor and a strong alkaline nature.
How prepare 6N ammonium hydroxide? The chemical name for NH4OH is ammonium hydroxide. Ammonia has alkaline properties and corrosive properties. This is also referred to as ammonia solution and is a solution comprised of ammonia in water. Name an amphoteric oxide reduced by the basic gas? Ammonia gas is easily compressed and under pressure forms a clear liquid. What does NH4OH decompose into? Find more answers Ask your question. Ingestion can cause diarrhoea, nausea, gastric pain and, in extreme cases, perforation, central nervous system agitation, shock, seizures and pulmonary oedema. Wiki User. Ammonium hydroxide is a base. More answers.

Ideal variant