Nitrogen trifluoride lewis structure
Nitrogen trifluoride NF3 is a colorless, nonflammable gas with routine usage in the microelectronics industry. It is an essential molecule in plasma science as an efficient fluorine source in manufacturing massive-scale integrated circuits. Although NF3 is indispensable in the electronics industry, it is a significant greenhouse gas, nitrogen trifluoride lewis structure, and its heat storage capacity is 17, times that of carbon dioxide.
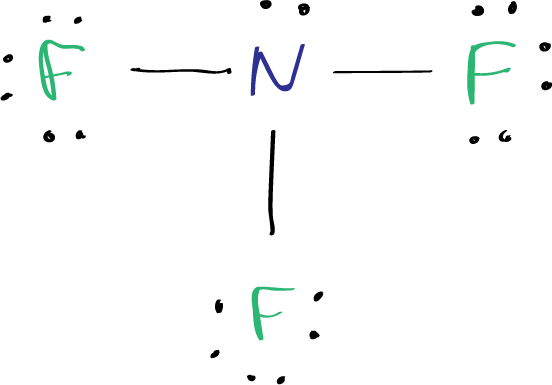
In the lewis structure of Nitrogen trifluoride NF 3 , there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. After drawing the lewis structure of NF 3 , you can decide shape of the NF 3 molecule. In the lewis structure of NF 3 , there are three N-F bonds and one lone pair on nitrogen atom which is the center atom.
Nitrogen trifluoride lewis structure
The NF3 molecule, composed of one nitrogen atom and three fluorine atoms, holds within its structure a fascinating arrangement of atoms and electrons that govern its chemical behavior. By delving into the principles of valence electrons, formal charges, and the octet rule, we can decipher the molecular puzzle that NF3 presents. Determine Total Valence Electrons. Begin by identifying the number of valence electrons for each atom in the NF3 molecule. Nitrogen N is in Group 15 of the periodic table and has 5 valence electrons, while Fluorine F is in Group 17 and possesses 7 valence electrons. Choose the Central Atom. In the NF3 molecule, nitrogen N is the least electronegative atom. As a result, it will serve as the central atom, with fluorine F atoms surrounding it. Connect Atoms with Electron Pairs. Connect the nitrogen N atom to each fluorine F atom with a single bond a pair of electrons. This forms three bonds, using 6 valence electrons. Place the remaining 20 valence electrons around the atoms as lone pairs.
Since the overall formal charge is zero, the above Lewis structure of NF3 is most appropriate, reliable, and stable.
The Lewis structure of nitrogen trifluoride NF 3 consists of three N-F bonds and a lone pair on the nitrogen atom, while each fluorine atom has three lone pairs. Drawing the Lewis structure of NF 3 involves several steps starting from the valence electrons of nitrogen and fluorine atoms, which are explained in detail in this tutorial. The Lewis structure of NF 3 displays a central nitrogen atom with three N-F bonds and one lone pair, while each fluorine atom has three lone pairs. In the Lewis structure of the NF 3 molecule, a central nitrogen atom is surrounded by three fluorine atoms. Each nitrogen — fluorine bond is represented by a single line, and there is a lone pair of electrons on the nitrogen atom. The three fluorine atoms are arranged in a trigonal pyramidal geometry such as ammonia molecule , with each fluorine atom positioned at a
Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Its noticeable characteristics include being colorless and carrying a musty or moldy odor. One of the main reasons why this chemical compound is an essential topic is because it is a greenhouse gas. It is said to have quite high global warming potential but a comparatively low value of radiative forcing. Other than being a greenhouse gas and contributing to the climatic change of the planet, NF3 has several applications. It is used to produce chemical fluoride lasers and remove silicon-based compounds during semiconductor manufacturing.
Nitrogen trifluoride lewis structure
In the lewis structure of Nitrogen trifluoride NF 3 , there are three N-F bonds and one lone pair on nitrogen atom. Each fluorine atom has three lone pairs. Lewis structure of NF 3 can be drawn by starting from valence electrons of nitrogen and fluorine atoms in several steps. Each step of drawing the lewis structure of NF 3 is explained in detail in this tutorial. After drawing the lewis structure of NF 3 , you can decide shape of the NF 3 molecule. In the lewis structure of NF 3 , there are three N-F bonds and one lone pair on nitrogen atom which is the center atom. You have to follow few steps to draw the lewis structure of NF 3. Because nitrogen trifluoride is a simple molecule, these steps are not complex and do not require all steps which are used to draw lewis structures of complex molecules and ions.
Scully truck rental
Nitrogen, with a maximum valence of 5, has a higher valence than fluorine, which has a maximum valence of 1. Additionally, nitrogen has a lower electronegativity value 2. It is essential to consider both the ability to have a greater valence and to be the most electropositive element. As a result, it will serve as the central atom, with fluorine F atoms surrounding it. Testing ammonia gas Sodium and ammonia reaction Ammonia and chlorine reaction NO 2 lewis structure. The central nitrogen atom achieves an octet of electrons by having two lone pairs and three bond pairs from the single bonds to fluorine atoms. Since the overall formal charge is zero, the above Lewis structure of NF3 is most appropriate, reliable, and stable. In the NF3 molecule, nitrogen N is the least electronegative atom. This forms three bonds between nitrogen and fluorine. In the lewis structure of NF 3 , there are three N-F bonds and one lone pair on nitrogen atom which is the center atom. Now, we can start to mark lone pairs on atoms. This satisfies the octet rule for the central atom. Those steps are explained in detail in next sections.
Transcript: Hi, this is Dr. We're going to do the Lewis structure for NF3, nitrogen trifluoride. On the periodic table, Nitrogen is in group 5 or 15, so it has 5 valence electrons; and then Fluorine is in group 7 or 17, it has 7.
To find out total valence electrons given by a particular element, you should multiply number of electrons of the valance shell by the number of atoms of that element. This indicates that Nitrogen and fluorine are chemically bonded with each other in an NF3 molecule. Step by step drawing the Lewis structure of NF 3 To draw the Lewis structure of NF 3 , a simple molecule, a few steps need to be followed, which are not as complex as those required for drawing the structures of more complex molecules and ions. This satisfies the octet rule for the central atom. Fluorine , a group VIIA element, has seven electrons in its valence shell, while nitrogen , a group VA element, has five electrons in its valence shell. The final Lewis structure for NF3 is as follows:. To evaluate the stability of the Lewis structure, check for any formal charges. Copy link. To draw the Lewis structure of NF 3 , a simple molecule, a few steps need to be followed, which are not as complex as those required for drawing the structures of more complex molecules and ions. Search for: Search Button.

I consider, that you are not right. Let's discuss it. Write to me in PM.
The excellent message))
I am sorry, that has interfered... This situation is familiar To me. I invite to discussion.