Noble gas configuration chart
Last Updated: December 11, Fact Checked. This article was co-authored by Bess Ruff, MA.
The content that follows is the substance of General Chemistry Lecture In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located.
Noble gas configuration chart
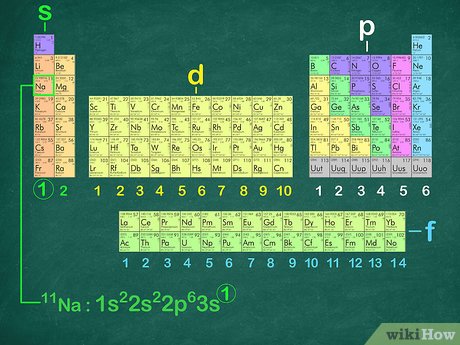
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go. The noble gases have the same problem—there is no room for any more electrons in their outer shells. They are completely full and cannot handle any more. Sodium, element number 11, is the first element in the third period of the periodic table. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. The elements that are found in the last column of the periodic table are an important group of elements called the noble gases. They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. Again, the number of valence electrons increases from one to eight across the third period. The fourth and subsequent periods follow the same pattern, except for the use of a different noble gas. All elements can be represented in this fashion. Search site Search Search. Go back to previous article.
This is called Hund's Rule: "Half fill before you Full fill" and again this rule was established based on energy calculations that indicated that this was the way atoms actually distributed their electrons into the orbitals. Answer, noble gas configuration chart, adding protons to the nucleus and adding electrons to the valence shell of the element. We will use neon for the noble gas configuration because it is in period 2.
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus element 15 as an example, the concise form is [Ne] 3s 2 3p 3. Here [Ne] refers to the core electrons which are the same as for the element neon Ne , the last noble gas before phosphorus in the periodic table. The valence electrons here 3s 2 3p 3 are written explicitly for all atoms. Electron configurations of elements beyond hassium element have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule.
Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration or Noble gas configuration as well as Full electron configuration is also mentioned in the table. Let me tell you how this Interactive Periodic Table will help you in your studies. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. You will get the detailed information about the periodic table which will convert a newbie into pro.
Noble gas configuration chart
As you have learned, the electron configurations of the elements explain the otherwise peculiar shape of the periodic table. Although the table was originally organized on the basis of physical and chemical similarities between the elements within groups, these similarities are ultimately attributable to orbital energy levels and the Pauli principle, which cause the individual subshells to be filled in a particular order. For example, the two columns on the left, known as the s block , consist of elements in which the ns orbitals are being filled. The six columns on the right, elements in which the np orbitals are being filled, constitute the p block. Within each column, each element has the same valence electron configuration—for example, ns 1 group 1 or ns 2 np 1 group As you will see, this is reflected in important similarities in the chemical reactivity and the bonding for the elements in each column. Because each orbital can have a maximum of 2 electrons, there are 2 columns in the s block, 6 columns in the p block, 10 columns in the d block, and 14 columns in the f block. Hydrogen and helium are placed somewhat arbitrarily. Although hydrogen is not an alkali metal, its 1 s 1 electron configuration suggests a similarity to lithium [He]2 s 1 and the other elements in the first column.
Island apk
In the d block, specifically the groups containing Chromium and Copper, there is an exception in how they are filled. However, a curious thing happens after the 3 p subshell is filled: the 4 s subshell begins to fill before the 3 d subshell does. The more electronegative the element, the higher the ionization eneregy. If the element is in the fourth row of the periodic table, it is in period four. Of those 7 electrons, 2 can go into the 3 s subshell, and the remaining 5 electrons can go into the 3 p subshell. Armaan Sidhu Jan 3, Skip to Content. The noble gas you will use will be located in period three. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes":. When writing some of the lower table configurations the total configuration can be fairly long. Create an account. The table below shows the scale values for the elements. Substitute the noble gas for the same number of electrons the noble gas has.
If you're seeing this message, it means we're having trouble loading external resources on our website.
Looking at the periodic table, you can see that Oxygen has 8 electrons. Recognize what the last orbital of each configuration will be. The fourth electron shell has an s, p, d, and f energy level. You just have to finish the configuration from where the noble gas leaves it:. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. BUT what we haven't discussed is how these orbitals get filled The Electron Affinity of an element is the amount of energy gained or released with the addition of an electron. If the element is in the fourth row of the periodic table, it is in period four. This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. But again the construction of the electron configuration gives us the answer. Then count which row the element is in. Read Edit View history.

It's just one thing after another.
I am am excited too with this question. Prompt, where I can find more information on this question?
I consider, that you are not right. Let's discuss it.