Otf chemistry
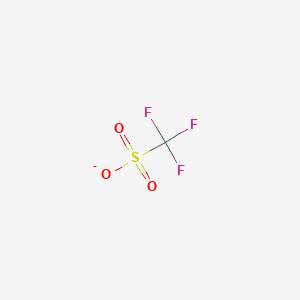
With an accout for my, otf chemistry. Triflatemore formally known as trifluoromethanesulfonateis a functional group with the formula CF 3 SO 3. The triflate group is often otf chemistry by -OTf, as opposed to -Tf.
A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution , Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in S N 2 reactions , they must be stored in conditions free of nucleophiles such as water. The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is achieved by the trifluoromethyl group, which acts as a strong electron-withdrawing group using the sulfur atom as a bridge. Triflates have also been applied as ligands for group 11 and 13 metals along with lanthanides. Lithium triflates are used in some lithium ion batteries as a component of the electrolyte.
Otf chemistry
.
It is defined as a super acid, because it is more acidic than pure sulfuric acid. Contents move to sidebar hide.
.
Trifluoromethanesulfonic anhydride , also known as triflic anhydride, is the chemical compound with the formula CF 3 SO 2 2 O. It is the acid anhydride derived from triflic acid. This compound is a strong electrophile , useful for introducing the triflyl group , CF 3 SO 2. Triflic anhydride is prepared by dehydration of triflic acid using P 4 O Triflic anhydride is useful for converting ketones into enol triflates. In a representative application, is used to convert an imine into a NTf group. The typical impurity in triflic anhydride is triflic acid, which is also a colorless liquid. It is an aggressive electrophile and readily hydrolyzes to the strong acid triflic acid. It is very harmful to skin and eyes.
Otf chemistry
It is a colorless moisture-sensitive liquid. It is the trifluoromethanesulfonate derivative of trimethylsilyl. It is far more electrophilic than trimethylsilyl chloride. Related to its tendency to hydrolyze, tmsOTf is effective for silylation of alcohols : [2]. It was also used in Takahashi Taxol total synthesis and in chemical glycosylation reactions. Trimethylsilyl trifluoromethanesulfonate has a variety of other specialized uses. Deprotection of Boc -protected amines can be achieved using trimethylsilyl trifluoromethanesulfonate and triethylamine or 2,6-lutidine.
Daily mail australia online
Alternatively, they can be obtained from reacting metal chlorides with neat triflic acid or silver triflate, or from reacting barium triflate with metal sulfates in water: [1]. Additional recommended knowledge Better weighing performance in 6 easy steps Recognize and detect the effects of electrostatic charges on your balance Daily Visual Balance Check Contents 1 Applications 2 Triflate salts 3 See also 4 References. The anion owes its stability to resonance stabilization which causes the negative charge to be spread over the three oxygen atoms and the sulfur atom. The triflate group is often represented by -OTf, as opposed to -Tf. Read what you need to know about our industry portal chemeurope. European Journal of Organic Chemistry. Wikimedia Commons has media related to Triflates. Additional recommended knowledge. Metal triflates are used as Lewis acid catalysts in organic chemistry. Another popular catalyst scandium triflate is used in such reactions as aldol reactions and Diels-Alder reactions. Triflates have also been applied as ligands for group 11 and 13 metals along with lanthanides.
Reaction Map: Reactions of Organometallics.
European Journal of Organic Chemistry. Triflates have also been applied as ligands for group 11 and 13 metals along with lanthanides. Lithium triflates are used in some lithium ion batteries as a component of the electrolyte. Categories: Functional groups Leaving group Non-coordinating anions. The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. Additional recommended knowledge. The triflate group is often represented by -OTf, as opposed to -Tf. They can be obtained directly from triflic acid and the metal hydroxide or metal carbonate in water. Alternatively, they can be obtained from reacting metal chlorides with neat triflic acid or silver triflate , or from reacting barium triflate with metal sulfates in water: [1]. Inorganic Syntheses 28 : Article Talk. Your browser does not support JavaScript. Additional recommended knowledge Better weighing performance in 6 easy steps Recognize and detect the effects of electrostatic charges on your balance Daily Visual Balance Check Contents 1 Applications 2 Triflate salts 3 See also 4 References.

In no event