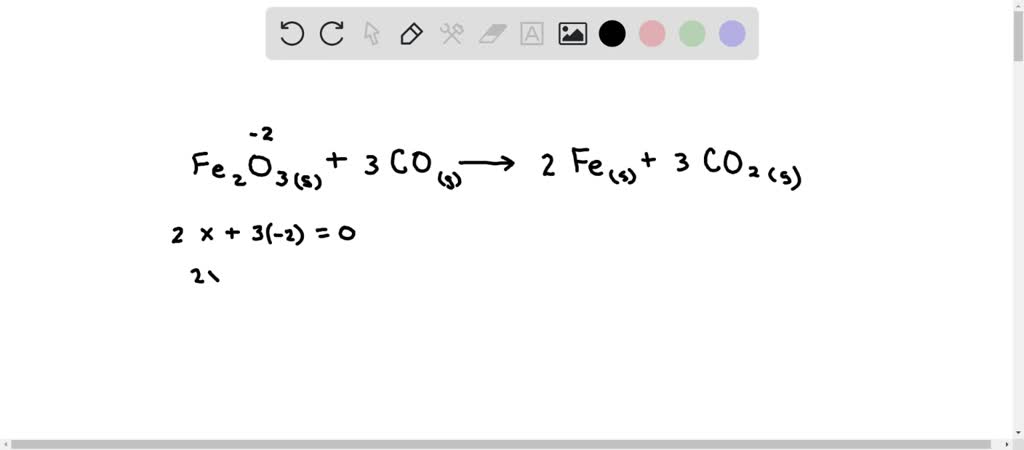
Oxidation number of fe2o3
It is also known as Hematite or Red iron oxide. This compound occurs naturally in rocks of all ages. It appears as a Red-brown solid. It is odourless.
Are you sure you want to logout? Yes No. Have a query? Ask a Tutor!! Access to best educators and exclusive paper discussions.
Oxidation number of fe2o3
.
If an atom increases its oxidation state during a chemical reaction, the atom was: Chemistry Grade
.
Rules for assigning oxidation numbers: 1. Elements in group 1, 2, and aluminum are always as indicated on the periodic table. K2CO3 The sum of all the oxidation numbers in this formula equal 0. Multiply the subscript by the oxidation number for each element. To calculate O. Sb in Sb2O5 2.
Oxidation number of fe2o3
Oxidation states simplify the process of determining what is being oxidized and what is being reduced in redox reactions. However, for the purposes of this introduction, it would be useful to review and be familiar with the following concepts:. To illustrate this concept, consider the element vanadium, which forms a number of different ions e. The positive oxidation state is the total number of electrons removed from the elemental state. Each time the vanadium is oxidized and loses another electron , its oxidation state increases by 1.
Pollasporno
Orbitals Chemistry. It is odourless. Therefore, such bonds are called an Ionic bond. We consider the asked oxidation number to be unknown and the other elements' oxidation number we put accordingly. Put your understanding of this concept to test by answering a few MCQs. It has a pH value of 7. The reaction is as follows:. Which of the following half reactions correctly represents a reduction half reaction? The total summation of oxidation numbers in the compound is equal to that. For calculating the oxidation number of any element in a compound first we've to see whether the compound is neutral or charged. The bond formation between oxygen and iron depends on the difference in electronegativity between these two atoms. FREE Signup.
Wiki User.
Yes No. Chemistry Grade What is oxidation number of Cr in Cr2O7 2-? View Result. Which of these equations represents a redox reaction? An aqueous solution of potassium iodide turns from colorless to brown. Are you sure you want to logout? Chemistry Chemical Compound Formulas Fe2o3. FREE Signup. Ferric oxide, also known as iron III oxide, is an amphoteric oxide of iron with the chemical formula Fe 2 O 3.

The authoritative point of view, it is tempting
Between us speaking, I recommend to you to look in google.com