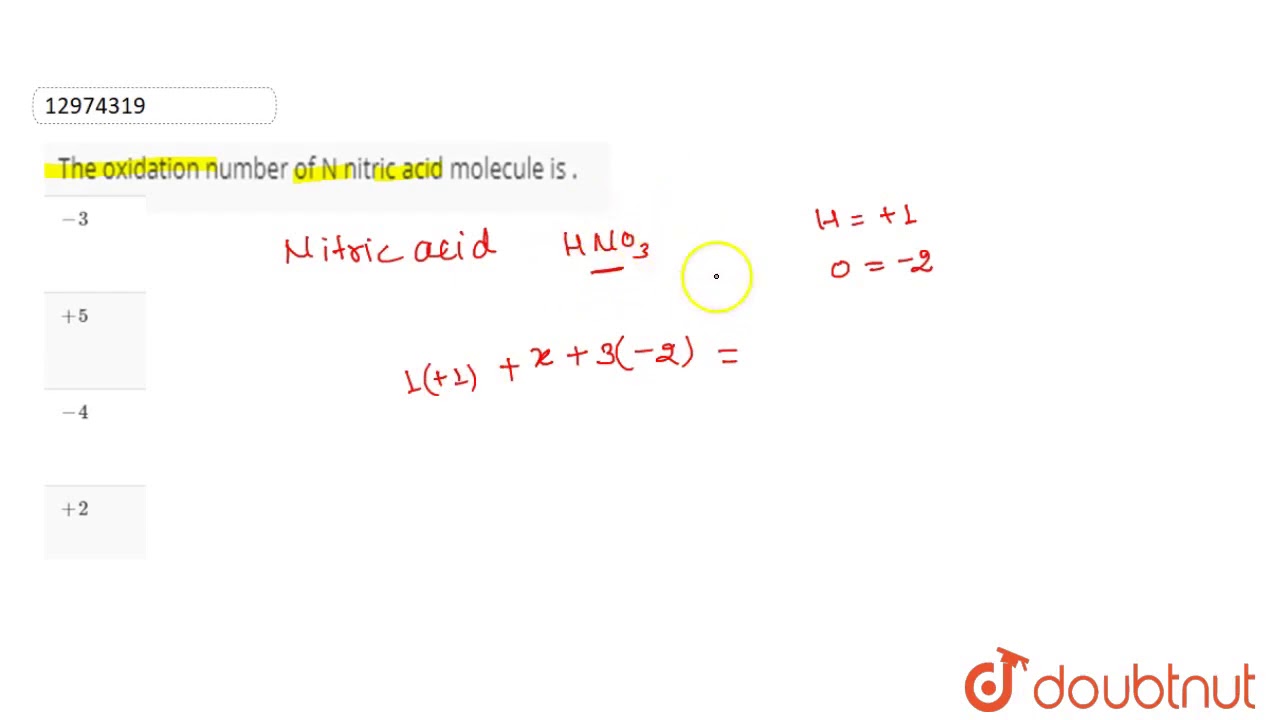
Oxidation number of n in hno3
What is the oxidation number of N in H N O 4? Nitrous oxide N 2 is.
In comparing the chemistry of the amines with alcohols and ethers, we discover many classes of related compounds in which nitrogen assumes higher oxidation states, in contrast to limited oxidation states of oxygen. In this context, keep in mind that the oxidation state of elemental oxygen O 2 and nitrogen N 2 is defined as zero. The most prevalent state of covalently bonded oxygen is This is the case for water, alcohols, ethers and carbonyl compounds. Because of the low covalent bond energy of the peroxide bond ca. The following table lists some of the known organic compounds of nitrogen, having different oxidation states of that element. Some of these classes of compounds have been described; others will be discussed later.
Oxidation number of n in hno3
Wiki User. There are two oxidation numbers. HNO3 already has hydrogen and nitrogen in their highest possible oxidation states. So sum is zero. It shows some oxidation numbers. No, the can only have positive oxidation numbers. All metals have positive oxidation numbers. Non-metals may have positive or negative oxidation numbers and some metalloids have both positive and negative oxidation numbers as well. Tags Oxidation Numbers Subjects. Log in. Study now See answer 1. Best Answer. Q: What is the oxidation numbers of HNO3?
View Solution. Is Nitrous an acid or base?
Wiki User. The name gives the clue. If any chemical is described as '-ous' it has the lower oxidation state. Hydrogen and nitrogen are both elements. That means that neither of them are made up of other elements.
If we multiply these by how many of that atom we have in the compound, the number left is. Oxygen is typically stackrel -II O , and it is so here And we remember our definition of oxidation numbers. Oxidation number is the charge left on the atom of interest when all the bonding pairs of electrons are broken, with the charge going to the MOST electronegative atom. Chemistry Electrochemistry Oxidation Numbers.
Oxidation number of n in hno3
What is the oxidation number of N in NO 3 -? Byju's Answer. Open in App. The oxidation state or oxidation number can be defined as the loss or gain of electrons from an atom, molecule, or ion to achieve a stable electronic configuration. Each element shows a different oxidation state depending upon the total number of valence shell electrons. Nitrogen is a chemical element with atomic number 7. The nitrate ion is having -1 charge on it and thus it is not a neutral atom.
Stranded hair extensions
Studying in Grade 6th to 12th? Because of the low covalent bond energy of the peroxide bond ca. This reaction is known as the Cope Elimination. The spin of the nitroxyl unpaired electron may be studied by a technique called electron paramagnetic resonance epr or esr. Predict whether the following redox reaction is feasible under the sta What is the oxidation number for HNO3? What is the oxidation state for ferrous chloride? Phosphorous Analogs of Amines Phosphorus is beneath nitrogen in the periodic table. Q: What is the oxidation state of an individual nitrogen atom in rm HNO3? How do oxidation numbers vary with the periodic table? For such a mechanism, the beta-hydrogen and amine oxide moieties necessarily have a syn-relationship. All Rights Reserved. The coordinate covalent N—O function is polar, with the oxygen being a powerful hydrogen bond acceptor.
Nitric acid and nitrous acid are mono basic oxoacids of nitrogen. But they are very different in chemical and physical properties.
It is commonly carried out by dropwise addition of an amine oxide solution to a heated tube packed with small glass beads. Thus, site-directed spin labeling SDSL has emerged as a valuable technique for mapping elements of secondary structure, at the level of the backbone fold, in a wide range of proteins, including those not amenable to structural characterization using classical structural techniques, such as nuclear magnetic resonance and X-ray crystallography. The coordinate covalent N—O function is polar, with the oxygen being a powerful hydrogen bond acceptor. What exactly is a Valence State? Why is the oxidation state of noble gas zero? What is no2 H2O? How do oxidation numbers vary with the periodic table? Amine oxides are not the only functions that undergo a unimolecular syn-elimination on heating. This is the case for water, alcohols, ethers and carbonyl compounds. Phosphorous Analogs of Amines Phosphorus is beneath nitrogen in the periodic table. Resources Leaderboard All Tags Unanswered.

))))))))))))))))))) it is matchless ;)
The properties leaves
It not so.