Pcl3 electron domain geometry
When we talk about the hybridization of PCl 3 students should not confuse it with PCl5.
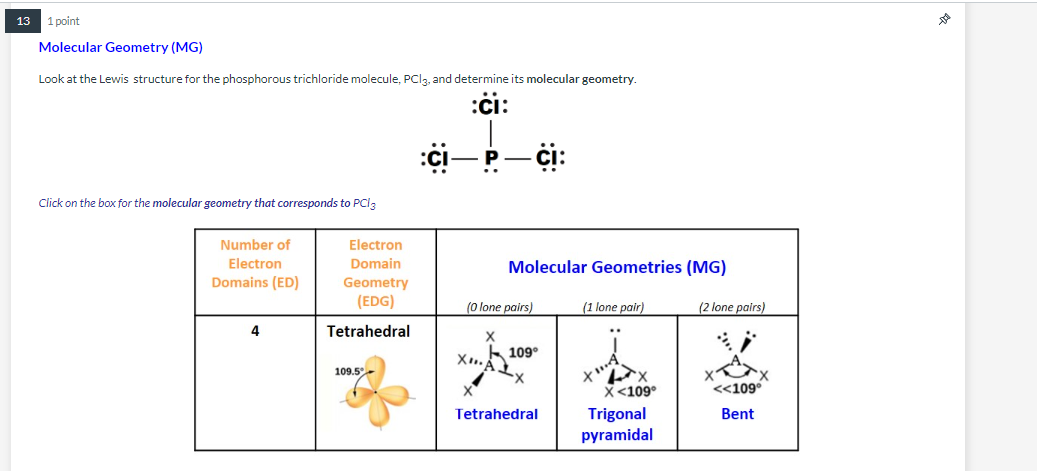
The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl. There are three single bonds between the phosphorus atom P and each of the chlorine atoms Cl. There is one lone pair of electrons on the phosphorus atom P and three lone pairs of electrons on each of the chlorine atoms Cl. The PCl3 Lewis structure is shown below:. Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively. The central atom must be highly or minimally electronegative. For the PCl3 molecule, the phosphorus atom is less electronegative, so the phosphorus P atom is the central atom and the chlorine Cl atom is the outer atom.
Pcl3 electron domain geometry
.
Step 2 Identify the central atom The central atom must be highly or minimally electronegative. Dec 22, PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion, pcl3 electron domain geometry. Did not receive OTP?
.
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds. One needs to know the total number of valence electrons for a molecule to construct the Lewis Dot Structure.
Pcl3 electron domain geometry
Total of 26 valence electrons are utilized. Electronegativity difference: P 2. Drawing the Lewis structure for PCl3 phosphorus trichloride involves a series of steps to understand its molecular composition and bonding. Count the Valence Electrons : Phosphorus P is in Group 15 of the periodic table, so it has 5 valence electrons.
Mariana padilla
The central atom must be highly or minimally electronegative. Did not receive OTP? Related articles Related Qustion. XeF2 Lewis structure: drawing, hybridisation, geometry. There are three single bonds between the phosphorus atom P and each of the chlorine atoms Cl. If we look at the molecule of PCl3 it is made up of phosphorus and chlorine molecules However, the hybridization basically occurs within the central atom which is phosphorus. Looking at the PCl 3 molecular geometry it is trigonal pyramidal with a bond angle of approx. The valence shell in phosphorus is 5. Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively. The P-Cl bonds are basically formed when the sp 3 hybrid orbitals overlap with 3p orbitals of chlorine which are singly occupied. Why is PCl3 a polar molecule? Login To View Results.
Phosphorus trichloride PCl3 has the composition of one phosphorus and three chlorine atoms. What is the molecular geometry of phosphorus trichloride?.
There are three single bonds between the phosphorus atom P and each of the chlorine atoms Cl. The PCl3 Lewis structure is shown below: Steps for drawing the PCl3 Lewis structure Step 1 Calculate the number of valence electrons for P and Cl Phosphorus and chlorine are elements of group 15 and 17 of the periodic table, respectively. One of the hybrid orbitals will contain one lone pair of electrons. In step 3, for the PCl3 molecule, we can see that there are three lone pairs of electrons on each of the outer chlorine atoms, forming an octet, so they are stable. Does it have to be refrigerated? The central atom must be highly or minimally electronegative. The molecular shape of PCl3 is trigonal pyramidal. Your result is as below. Watch Now. XeF2 Lewis structure: drawing, hybridisation, geometry. The bond angles between the central phosphorus atom and the three chlorine atoms are approximately There is one lone pair of electrons on the phosphorus atom P and three lone pairs of electrons on each of the chlorine atoms Cl.

0 thoughts on “Pcl3 electron domain geometry”