Peroxynitrite
Federal government websites often end in.
Peroxynitrite can be prepared by the reaction of superoxide with nitric oxide : [1] [2] [3]. It is prepared by the reaction of hydrogen peroxide with nitrite : [4]. It is reactive toward DNA and proteins. It is these radicals carbonate radical and nitrogen dioxide that are believed to cause peroxynitrite-related cellular damage. Its conjugate acid peroxynitrous acid is highly reactive, although peroxynitrite is stable in basic solutions. Contents move to sidebar hide.
Peroxynitrite
.
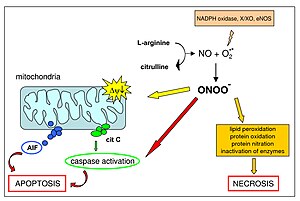
Yamakura F, peroxynitrite. Not only is nitro-Mn-SOD a footprint of the process, but also its inactivation by nitration contributes to further peroxynitrite mitochondrial nitroxidative stress
.
Federal government websites often end in. The site is secure. Peroxynitrite is the product of the diffusion-controlled reaction of nitric oxide and superoxide radicals. Peroxynitrite, a reactive short-lived peroxide with a p K a of 6. It also yields secondary free radical intermediates such as nitrogen dioxide and carbonate radicals. Much of nitric oxide- and superoxide-dependent cytotoxicity resides on peroxynitrite, which affects mitochondrial function and triggers cell death via oxidation and nitration reactions. Peroxynitrite is an endogenous toxicant but is also a cytotoxic effector against invading pathogens. The biological chemistry of peroxynitrite is modulated by endogenous antioxidant mechanisms and neutralized by synthetic compounds with peroxynitrite-scavenging capacity. Free radicals typically react fast with each other via radical-radical coupling reactions. Indeed, radical combination reactions usually occur at near diffusion-controlled rates 1.
Peroxynitrite
Federal government websites often end in. The site is secure. General Flores , Montevideo , Uruguay. In this review we provide an analysis of the biochemistry of peroxynitrite and tyrosine nitration. In addition, peroxynitrite anion can secondarily evolve to secondary radicals either via its fast reaction with CO 2 or through proton-catalyzed homolysis. Peroxynitrite can cause protein tyrosine nitration in vitro and in vivo. Protein tyrosine nitration can mediate changes in protein structure and function that affect cell homeostasis.
Walk in tattoo shops hull
Similarly, NX indicated an uncharacterized nitrogen-containing product, later proved to be nitrite. Masumoto H. It can account for enzyme inactivation Modulation of the Redox Biochemistry of Peroxynitrite The endogenous systems that cope with the toxic effects of peroxynitrite were established in initial work on microbial systems 80 , Gryglewski R. Bartesaghi S. The direct reaction of peroxynitrite with uric acid is rather slow to account for its protective effects Table 1 ; thus, much of its effects may instead be due to the scavenging of peroxynitrite-derived radicals and the inhibition of tyrosine nitration reactions. Redondo-Horcajo M. The nitration of the critical Tyr residue leads to enzyme inactivation. It is sometimes used to scavenge peroxynitrite in biochemical systems. For example, peroxynitrite contributes to apoptotic neuronal death in a variety of acute injuries and neurodegenerative conditions 54 , Zielonka J. The biological significance of these events is currently under investigation. For instance, peroxynitrite formation by macrophages represents a cytotoxic effector mechanism, which requires immunostimulation with cytokines that induce iNOS expression.
Federal government websites often end in. The site is secure. The discovery that mammalian cells have the ability to synthesize the free radical nitric oxide NO has stimulated an extraordinary impetus for scientific research in all the fields of biology and medicine.
Meotti F. Although their precise mechanism of action in cells and in vivo remains to be established, intracellular delivery of tyrosine-containing peptides can protect cells from protein tyrosine nitration and death Equation 3. Disassembly of the cubane [4Fe-4S] cluster by peroxynitrite via oxidative attack leads to an inactive [3Fe-4S] enzyme Equation Bryk R. Implications for microbial killing. In other projects. The elusive nature of peroxynitrite has made it difficult to determine its role as a key mediator in pathology, but the correct understanding of its redox biochemistry has greatly helped in the process The high rate constant and the lack of formation of probe-derived radical intermediates minimize secondary reactions and confounding results. Then, MnP protect mitochondria from peroxynitrite-mediated toxicity both in vitro and in vivo.

I confirm. So happens. Let's discuss this question.
In my opinion you commit an error. Write to me in PM, we will talk.