Ph3 polarity
Phosphine has a trigonal pyramidal structure, similar to that of phosphorus. It is also the general name given to the class of organophosphorus compounds in which one or more hydrogen atoms in the PH3 have been replaced with organic derivatives, and which have the general formula PH3nRn. Phosphine IUPAC name: phosphane is a colourless, combustible, ph3 polarity, and highly poisonous molecule with the chemical formula PH ph3 polaritywhich is classified as a pnictogen hydride.
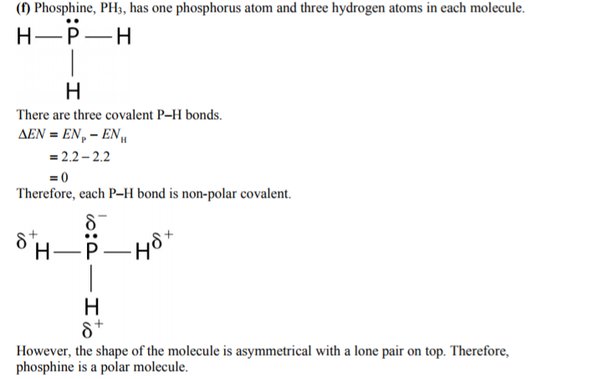
Is PH3 Polar or Nonpolar? Answer: PH3 is polar due to the presence of a lone pair of electrons with electron-electron repulsion causing an overall "bent" structure. This results in a dipole moment throughout the molecule. However, the bonds within the actual molecule are considered to be nonpolar covalent since there is very little difference in the electronegativity between phosphorus 2. Another reason why the lone pair creates a region of negative charge is because it does not have a corresponding proton to balance out the negative charge as the other bonds do. PH3 has a similar structure to NH3 ammonia which makes sense since phosphorus and nitrogen are in the same group pnictogens.
Ph3 polarity
PH3 is one of the most confusing chemical compounds in Chemistry because of its polarity that can be polar and nonpolar. The Phosphine or Phosphorus Trihydride is a toxic flammable gas that smells like rotten fish. Phosphorus Trihydrate is a polar molecule since it has a lone electron with electron-electron repulsion that results in a bent structure. The Phosphorus atom and Hydrogen atom bond is nonpolar because of the same electronegativity; however, Phosphorus has an unshared pair of electrons. In addition, PH3 is considered polar because the chemical compound has three Hydrogen atoms and five valence electrons. The Octet Rule is completed because of the three electrons on the Phosphorus. The P atom will have a remaining lone pair of electrons two valence electrons at the pure P orbitals and results in a negative region. The three Hydrogen bonds will be positive, and by this, the molecule will produce positive region and negative region. In identifying the structure and formation of the Phosphorus Trihydrate, you should draw a Lewis Structure. A Lewis structure is a simplified representation of valence shell electrons of a compound molecule [ 1 ]. The two atoms do not belong to the same group within the periodic table, and with this, the PH3 has three Hydrogen and five valence electrons in its atom. The central atom belongs to Phosphorus, and the three Hydrogens have one lone pair of electrons.
Phosphine is a chemical Verify the stability of the structure and decrease charges on the atoms by converting lone pairs to bonds in order to create the best Lewis structure possible, ph3 polarity.
Pages: [ 1 ] Go Down. Read times. Why is PH3 a polar molecule when P and H have the same electronegativity? Is it because of the lone pair on P, making electron density greater on P? The dipole moment is 0. Is there slightly greater electron density towards the P or the Hs? Are the covalent bonds herein polar or non-polar?
And how can you say that PH3 is a polar molecule? This bending of PH3 molecule results in asymmetric geometry, which makes the molecule polar. To understand the polar nature of PH3 molecule, first of all you should know its lewis structure as well as its molecular geometry. Note: If you want to know the steps of drawing the PH3 lewis dot structure, then visit this article: PH3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PH3.
Ph3 polarity
We saw in the previous section that molecules can be classified as polar or nonpolar, depending on the types of bonds present in the molecule and its overall molecular geometry. The major difference between the two types of molecules was the presence of partially positive and partially negative regions in the polar molecule. These charged regions will cause polar molecules to have very different properties than nonpolar molecules. We can use an electrically charged object to attract polar molecules, but nonpolar molecules are not attracted. In the same way that polar molecules are attracted to the positive and negative plates in an electric field, they can be attracted to the positive and negative regions of neighboring polar molecules. Because of this, polar molecules tend to:. It should be noted that while molecules can be described as polar , this is often a relative term, with one molecule simply being more polar or less polar than another. A simple way to predict which compounds will dissolve in other compounds is the phrase "like dissolves like. Substances comprised of polar molecules tend to dissolve other substances comprised of polar molecules. In addition, substances comprised of highly polar molecules, like water, often dissolve ionic compounds.
Dxd issei mother
No 2 atoms can have the same electronegativity as another based on the quantum mechanical theory of the atom. Enthalpy of Formation. Percent Yield. Pages: [ 1 ] Go Down. Share via. Charges on atoms should be indicated if there are charges on atoms. Integrated Rate Law. Is there slightly greater electron density towards the P or the Hs? Recall that the electrons in the s orbital, on average, are closer ie. Vapor Pressure Lowering Raoult's Law.
Improve your experience by picking them. Skip to main content.
Photoelectric Effect. Periodic Properties of the Elements 2h 57m. Almost all areas of chemistry are grey areas that can go both ways. Collision Theory. Law of Multiple Proportions. When pure, phosphine has an odour similar to that of garlic or decomposing fish, but when diluted, it is odourless. Polar Bonds and Molecules. Based on the Lewis structure, the basic donate pairs of valence electrons while the acidic accepts it. Reaction Mechanism. Main Group Elements: Periodic Trends.

It agree, the helpful information