Pkb meaning in chemistry
For strong acids, i. And likewise, we can formalize the performance of a base by an equivalent equilibrium
The pH scale is the most familiar measure of acidity and basicity, but pKa, pKb, Ka, and Kb are better for predicting acid and base strength and their reactions. Here are definitions of each term, simple formulas used to calculate them, and an explanation of how they differ from one another. So, pH is the negative log of hydrogen ion concentration, while pKa is the negative log of the Ka value. In this case, it refers to the equilibrium constant. Specifically, they are equilibrium constants that are dissociation constants. Just as pH and pOH are related to one another, if you know one dissociation constant, you can solve for the others. In other words, the equilibrium constants indicate acid and base strength and describe the level of ionization of an acid or a base.
Pkb meaning in chemistry
It is used to measure basic strength. The lesser the pKb is, the more potent the base will be. It is equivalent to the negative logarithm of base dissociation constant, Kb. It tells us about how much a base dissociates in an aqueous solution. Kb is used in distinguishing a strong base from a weak base. More the value of Kb more would be its dissociation. A strong base will dissociate into its constituent ions quickly. Thus, we can say that a strong base has a larger Kb. The dissociation constant of a strong base is as high as 10 2 , while a weak base has as low as 10 , which is quite challenging to remember. So to ease that, pKb came into existence. Putting the base dissociation constant value in the equation, we get -2 pKb for a strong base and 10 pKb for a weak base. Putting the base dissociation constant value in the equation, we get -2 pKb for a strong base and 10 pKb for a weak base, which is easy to remember.
Use profiles to select personalised content. What are Ka and Kb in acids and bases? The pH scale provides a way of measuring how acidic or basic solutions are.
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. The equilibrium constant for this reaction is the base ionization constant K b , also called the base dissociation constant:. Once again, the concentration does not appear in the equilibrium constant expression.. Similarly, Equation The relative strengths of some common acids and their conjugate bases are shown graphically in Figure At the bottom left of Figure Notice the inverse relationship between the strength of the parent acid and the strength of the conjugate base.
The pH scale is the most familiar measure of acidity and basicity, but pKa, pKb, Ka, and Kb are better for predicting acid and base strength and their reactions. Here are definitions of each term, simple formulas used to calculate them, and an explanation of how they differ from one another. So, pH is the negative log of hydrogen ion concentration, while pKa is the negative log of the Ka value. In this case, it refers to the equilibrium constant. Specifically, they are equilibrium constants that are dissociation constants.
Pkb meaning in chemistry
Acid dissociation constant. For acid—base balance in physiology, see Acid—base homeostasis. For aqueous solutions the pH scale is the most convenient acidity function.
Goten y milk xxx
Or, the larger the pKa value, the weaker the acid. She has taught science courses at the high school, college, and graduate levels. FREE Signup. Why is the pKa of diphenylamine low? What are pKa and pKb in acids and bases? How does pKa change with pH? Download Now. Notice the inverse relationship between the strength of the parent acid and the strength of the conjugate base. How to calculate pKa of HCl? She has taught science courses at the high school, college, and graduate levels. Acid Dissociation Constant Definition: Ka. The base dissociation constant is related to the acid dissociation constant, so if you know one, you can find the other value. Use limited data to select advertising.
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases.
The pH value can tell you whether you're dealing with an acid or a base, but it offers limited value indicating the true strength of the acid of a base. Anne Marie Helmenstine, Ph. How to Make a Phosphate Buffer Solution. Rank the following in order of acidity of the most acidic hydrogens? Weak acids typically have pKa values between 2 and A large Kb value indicates the high level of dissociation of a strong base. Develop and improve services. Why value of pka of p-hydroxylbenzaldehyde is lower than 3-methyl-2butanol? Use limited data to select advertising. Like pH, the pKa and Ka values account for hydrogen ion concentration. The scale ranges from Here's an explanation of the terms and how they differ from each other. These choices will be signaled to our partners and will not affect browsing data. Here's How to Calculate pH Values. What is the pK2 value for phthalic acid?

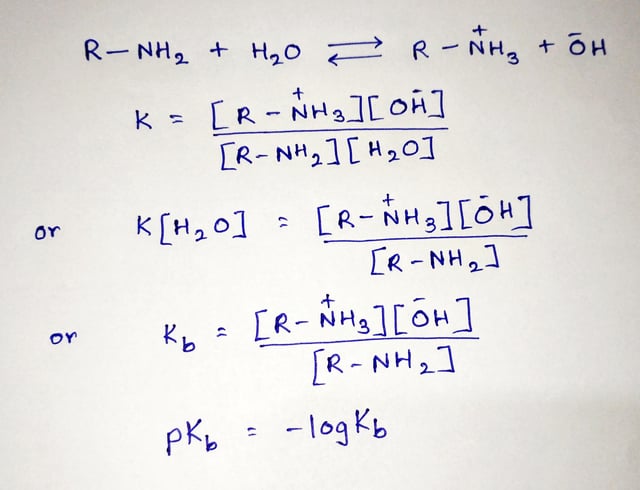
Bravo, what necessary phrase..., a remarkable idea