Quinoxaline
Quinoxaline C8H6N2commonly called 1,4-diazanaphthalene, 1,4-benzodiazine, quinoxaline, or benzopyrazine, is a very potent nitrogenous heterocyclic moiety consisting of quinoxaline benzene ring fused with the pyrazine ring. A number of different methods for the synthesis of quinoxaline derivatives have been reported in the literature, quinoxaline, but the most effective method, commonly used for the synthesis quinoxaline quinoxaline analogues involves the condensation of substituted o-phenylenediamines with 1, 2- dicarbonyl compounds in the presence of different catalyst s. The presence of different types of catalysts and their concentration affects the overall yield of the product.
A quinoxaline , also called a benzopyrazine , in organic chemistry , is a heterocyclic compound containing a ring complex made up of a benzene ring and a pyrazine ring. It is isomeric with other naphthyridines including quinazoline , phthalazine and cinnoline. Although quinoxaline itself is mainly of academic interest, quinoxaline derivatives are used as dyes, pharmaceuticals such as varenicline , and antibiotics such as olaquindox , carbadox , echinomycin , levomycin and actinoleutin. They can be formed by condensing ortho - diamines with 1,2- diketones. The parent substance of the group, quinoxaline, results when glyoxal is condensed with 1,2-diaminobenzene. One study [6] used 2-iodoxybenzoic acid IBX as a catalyst in the reaction of benzil with 1,2-diaminobenzene:. The antitumoral properties of quinoxaline compounds have been of interest.
Quinoxaline
Bioinspired ortho -quinone catalysts have been applied to oxidative synthesis of benzimidazoles, quinoxalines and benzoxazoles from primary amines in high yields under mild conditions with oxygen as the terminal oxidant. Zhang, Y. Qin, L. Zhang, S. Luo, Org. Bains, V. Singh, D. Adhikari, J. Shee, D. Panja, S. Kundu, J. Aerobic oxidation of deoxybenzoins is efficiently catalyzed by 1,4-diazabicyclo[2. The process has been successfully extended to a one-pot synthesis of quinoxalines from benzyl ketones and aromatic 1,2-diamines.
This method shows wide substrate scope and good functional group tolerance and provides a wide range of quinoxalines in good quinoxaline. Gayakhe, I. The Royal Society of Chemistry.
.
Federal government websites often end in. The site is secure. Quinoxalines, a class of N -heterocyclic compounds, are important biological agents, and a significant amount of research activity has been directed towards this class. They have several prominent pharmacological effects like antifungal, antibacterial, antiviral, and antimicrobial. Quinoxaline derivatives have diverse therapeutic uses and have become the crucial component in drugs used to treat cancerous cells, AIDS, plant viruses, schizophrenia, certifying them a great future in medicinal chemistry. Due to the current pandemic situation caused by SARS-COVID 19, it has become essential to synthesize drugs to combat deadly pathogens bacteria, fungi, viruses for now and near future. Since quinoxalines is an essential moiety to treat infectious diseases, numerous synthetic routes have been developed by researchers, with a prime focus on green chemistry and cost-effective methods.
Quinoxaline
Federal government websites often end in. The site is secure. Background: In recent decades, several viruses have jumped from animals to humans, triggering sizable outbreaks. Suitably functionalized polysubstituted quinoxalines show very interesting biological properties antiviral, anticancer, and antileishmanial , ensuring them a bright future in medicinal chemistry. Objectives: Focusing on the promising development of new quinoxaline derivatives as antiviral drugs, this review forms part of our program on the anti-infectious activity of quinoxaline derivatives.
Pop songs in the key of g
Yuan, K. Cui, Org. Adhikari, J. S2CID Yang, Z. Bioinspired ortho -quinone catalysts have been applied to oxidative synthesis of benzimidazoles, quinoxalines and benzoxazoles from primary amines in high yields under mild conditions with oxygen as the terminal oxidant. Cambridge University Press. Chen, Y. EC Number. A large number of quinoxaline analogues possessing different biological activities and their synthetic procedures have been patented worldwide. ISBN Xie, W. Trentin, C.
Quinoxaline has become a subject of extensive research due to its emergence as an important chemical moiety, demonstrating a wide range of physicochemical and biological activities. The last few decades have witnessed several publications utilizing quinoxaline scaffolds for the design and development of numerous bioactive molecules, dyes, fluorescent materials, electroluminescent materials and organic sensitizers for solar cell applications and polymeric optoelectronic materials.
This reactive zwitterion could react in situ with various trapping agents to furnish a range of structurally diverse quinoxalines. A large number of quinoxaline analogues possessing different biological activities and their synthetic procedures have been patented worldwide. Kim, C. One-pot two-step cyanide-mediated sequential reactions of ortho -phenylenediamines with aldehydes under aerobic oxidation conditions afford 2-aminoquinoxalines in high yields. Schulzke, A. Cheon, J. Chemical formula. Signal word. Article Talk. Precautionary statements. Gorden Yuan, K. Toggle limited content width.

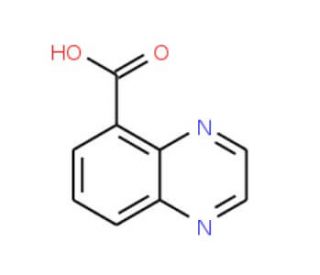
Very useful idea
Idea good, it agree with you.
What remarkable topic