Sf6 dot and cross diagram
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule.
Q: On your paper, draw a correct Lewis structure for the following molecule, where element E is in…. It is…. Starting from this structure, complete…. A: The lewis structure of H2SO3 can be drawn as follows;. Q: Cyanogen CN 2 is known as pseodohalogen because it has some properties like halogens. A: since you have posted a question it contains multiple subparts,we are entitled to answer only first….
Sf6 dot and cross diagram
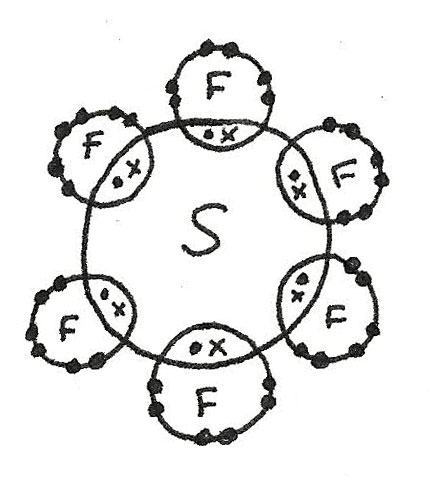
Covalent and dative sometimes called co-ordinate bonds occur between two or more non-metals, e. But what actually are they? A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. They are found in molecular elements or compounds such as chlorine or sulfur, but also in macromolecular elements and compounds like SiO2 and graphite. Single covalent bonds have just one shared pair of electrons. Regularly, each atom provides one unpaired electron the amount of unpaired electrons is usually equal to the number of covalent bonds which can be made in the bond. Dot and cross diagrams represent the arrangement of electrons in covalently bonded molecules. A shared pair of electrons is represented by a dot and a cross to show that the electrons come from different atoms. Unpaired electrons are used to form covalent bonds as previously mentioned. The unpaired electrons in orbitals of one atom can be shared with another unpaired electron in an orbital but sometimes atoms can promote electrons into unoccupied orbitals in the same energy level to form more bonds. This does not always occur, however, meaning different compounds can be formed - PCl3 and PCl4 are examples of this. An example where promotion is used is in sulfur hexafluoride SF6. The regular configuration of sulfur atoms is 1s2 2s2 2p6 3s2 3p4.
Use an arrow to show the direction of In this molecule, the sulfur has expanded its octet to 12 electrons.
They repel each other equally. It is a linear molecule. Carbon dioxide has two double bonds. There are two groups of two bonding pairs of electrons around the central carbon atom. The groups of electrons repel each other equally, so carbon dioxide is also a linear molecule.
SF 6 is an inorganic colorless greenhouse non-flammable gas with an octahedral geometry in which one sulfur atom is attached with six fluorine atoms. It has an orthorhombic crystalline structure and hypervalent in nature. In SF 6 , the S-F single bond length is Lewis structure known as electron dot structure was first introduced by eminent scientist Gilbert. Lewis structure has a great significance in Chemistry because number of bonds, nonbonding as well as bonding electrons, structure can be predicted from this structure.
Sf6 dot and cross diagram
Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among chemistry enthusiasts. Henri Moissan discovered the existence of SF6. Incidentally, he is also the discoverer of fluorine. The standard way of synthesizing SF6 is to expose S8 to F2. This method causes the formation of a few sulfur fluorides, but those can be eliminated through heating and then using NaOH to remove any additional SF4 molecules. SF6 cannot be used immediately after synthesis.
Hub by premier inn london kings cross
The compounds asked for in the syllabus are: Sulphur dioxide The dot-and-cross diagram showing only the outer electrons looks like this: This is quite awkward to draw tidily, and is better represented by a drawing with lines showing the pairs of shared electrons - but if you do that, you need to include the lone pair at the bonding level. Prior to protonation, you have five crosses of nitrogen's valence electrons and three dots for the bonding hydrogen electrons. This is because of the extra repulsion caused by the lone pairs. So starting off by drawing the Lewis structure:. Draw a dot and cross diagram to show the bonding in this ion. The four unused valency electrons form the two lone pairs. Since sulfur now has more than 8 electrons, we say that it "expands octet". See similar textbooks. For the…. NH3 has 4 pairs of e- around central atom 3bonding and 1 lone so is trigonal pyramidal with bond angles of ; BF3 only has 3 pairs so trigonal planar with angles of The composite structure is called a resonance hybrid. Melting and boiling points are high: it takes a lot of energy to break the large number of strong covalent bonds in the network of atoms.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding.
SF 2 is similar with a bond angle of 98 o but this greatly lowered angle is not simply to do with the effect of lone pair - lone pair repulsion. CF4 ii. When lone pairs are present, the letter E x is added. BF3 II. Two groups of electrons around the central atom two bonding pairs of electrons single bonds or two double bond pairs give a linear shape and bond angle of o In these examples the electron pair geometry is the same as the molecular geometry. SbCl 5 2 -. For the molecule SiBr2H2, answer the following: a number of valence electrons in the molecule…. You should read a part of the page which looks at co-ordinate dative covalent bonding. Calcium: 1s2 2s2 2p6 3s2 3p6 4s2, not 1s2 2s2 2p6 3s2 4s2 3p6. From left to right, EN will increase. This is the corresponding displayed formula of SF 6. Bonding pairs 4 H Lone pairs 0 BUT , larger X atoms or more bulky X groups might override the valence shell electron pair rule of 2. Its three lone pairs of electrons are arranged in equatorial positions, forming a linear ion.

It is absolutely useless.
In my opinion you are not right. I suggest it to discuss.