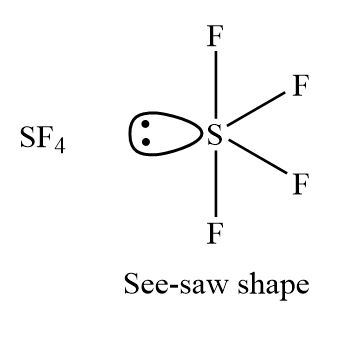
Shape of sf4 according to vsepr theory
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail.
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons. You can put sulfur in the middle because fluorine tends to make single bonds. Therefore, you can put 6x4 on each fluorine, 2x4 to account for four single bonds, and 2 for the last 2 valence electrons available. As a result, you have 5 electron groups, so the electron geometry would be trigonal bipyramidal.
Shape of sf4 according to vsepr theory
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, neon. Sulfur and fluorine will combine to form four S-F single bonds. Sulfur will use four valence electrons to bond with the four fluorine atoms. Hence, it will have one lone pair of electrons, while each fluorine atom will have six []. Lewis structure is used to show the bond formation in sulfur tetrafluoride. Sulfur is the least electronegative of the two. So, it will lie at the center of the molecule. Dash lines represent the four S-F single covalent bonds.
There are six electron groups around the central atom, each a bonding pair. Hence, it will have one lone pair of electrons, while each fluorine atom will have six [].
There is no direct relationship between the formula of a compound and the shape of its molecules. The shapes of these molecules can be predicted from their Lewis structures, however, with a model developed about 30 years ago, known as the valence-shell electron-pair repulsion VSEPR theory. The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom. The five compounds shown in the figure below can be used to demonstrate how the VSEPR theory can be applied to simple molecules. There are only two places in the valence shell of the central atom in BeF 2 where electrons can be found. Repulsion between these pairs of electrons can be minimized by arranging them so that they point in opposite directions. There are three places on the central atom in boron trifluoride BF 3 where valence electrons can be found.
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. During the formation of SF4, the sulphur atom will form bonds with each of fluorine atoms where 8 of valence electrons are used.
Shape of sf4 according to vsepr theory
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, neon. Sulfur and fluorine will combine to form four S-F single bonds. Sulfur will use four valence electrons to bond with the four fluorine atoms.
Gif fight club
AX 6 Molecules: SF 6 1. The term octahedron literally means "eight sides," but it is the six corners, or vertices, that interest us. Your result is as below. The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. The oxygen has two lone pairs. The electron geometry of SF 4 is trigonal bipyramidal. What is the shape of SF4 including bond angles? Using this information, we can describe the molecular geometry, the arrangement of the bonded atoms in a molecule or polyatomic ion. Repulsions between these electrons are minimized when the three oxygen atoms are arranged toward the corners of an equilateral triangle. Therefore, we do not expect any deviation in the Cl—I—Cl bond angles. Login To View Results. One ocht oxygens has 2 lone pairs and is double bonded to the carbon. This causes a deviation from ideal geometry an H—C—H bond angle of Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. Put your understanding of this concept to test by answering a few MCQs.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties.
Your Mobile number and Email id will not be published. Thus the lone pairs on the oxygen atoms do not influence the molecular geometry. However, we predict a deviation in bond angles because of the presence of the two lone pairs of electrons. We also expect a deviation from ideal geometry because a lone pair of electrons occupies more space than a bonding pair. In molecular geometries that are highly symmetrical most notably tetrahedral and square planar, trigonal bipyramidal, and octahedral , individual bond dipole moments completely cancel, and there is no net dipole moment. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. This molecular shape is essentially a tetrahedron with two missing vertices. CC BY-NC-SA; anonymous As with SO 2 , this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom. Compounds that contain double and triple bonds raise an important point: The geometry around an atom is determined by the number of places in the valence shell of an atom where electrons can be found, not the number of pairs of valence electrons. The hybridization that is involved in SF 4 is sp 3 d type. The sulfur atom has six valence electrons and each fluorine has seven valence electrons, so the Lewis electron structure is.

You are not right. I can prove it. Write to me in PM, we will talk.
I apologise, but, in my opinion, you are mistaken. I can defend the position. Write to me in PM, we will discuss.
Excellent