Sif4 molecular geometry
Mention the shape of NH3 and H2O. What is the expected geometry of AB5 molecule? View Solution.
Wiki User. Silicon Tetrafluoride has a tetrahedral molecular geometry. That means there are 4 F atoms around the central atom Si. It's geometry is tetrahedral. Molecular because it is between 2 non-metals.
Sif4 molecular geometry
.
Bond angle in P F 3 greater than the bond angle in P H 3 due to steric crowding as the size of F is larger than the size of H.
.
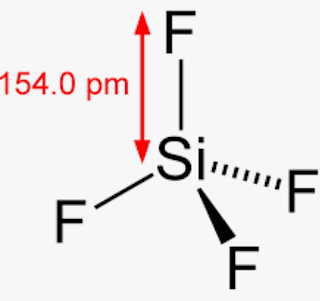
Drawing and predicting the SiF4 Molecular Geometry is very easy. Here in this post, we described step by step method to construct SiF4 Molecular Geometry. A three-step approach for drawing the SiF4 molecular can be used. The first step is to sketch the molecular geometry of the SiF4 molecule, to calculate the lone pairs of the electron in the central silicon atom; the second step is to calculate the SiF4 hybridization, and the third step is to give perfect notation for the SiF4 Molecular Geometry. The SiF4 Molecular Geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the SiF4 molecule. Finally, you must add their bond polarities to compute the strength of the Si-F bond dipole moment properties of the SiF4 Molecular Geometry. As a result, it has no dipole moment indefinitely. The SiF4 molecule has no dipole moment due to an equal charge distribution of negative and positive charges. Because the centre atom, silicon, has four Si-F bonds with the four fluorine atoms surrounding it. The F-Si-F bond generates a degree angle in a tetrahedral geometry.
Sif4 molecular geometry
The chemical formula for Silicon tetrafluoride is SiF4. It is a colorless gas with a pungent odor and is highly toxic. Silicon tetrafluoride is commonly used in the semiconductor industry as a source of silicon for deposition onto surfaces.
Closest auto parts store
The bond angle in F2O is smaller than the bond angles in NF3. There is no possibility of formation of double bonds in PH3. What is the molecular geometry of secl2? Bond angle in P F 3 greater than the bond angle in P H 3 due to steric crowding as the size of F is larger than the size of H. The purpose of increasing F-A-F bond angles is to reduce the …Bonding with grandparents is important, whether they live far away or next door. Large atoms, such as Cl or Br, will cause bond angles to deviate from the ideal, and the presence of a multiple bond will cause a deviation as well owing to the increased electron density, so CH3Br should deviate from the idealized bond angle. Which of the following has the largest bond angle? Each fluorine atom has three lone pairs. Ball, Edward Mercer. Best answer NF3 has three F atoms bonded to a central N atom with one lone electrons pair on the N Hybridization: The bond angle is dependent on the hybridization of the central atom of the compound. Fluorine is more electronegative than hydrogen and the electron density in the N-F bond is skewed towards the fluorine. Study now See answer 1.
Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula Si F 4. It was first prepared in by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid.
How many of the following cannot be hydrolyzed? Select the answer that represents the correct parent structure for each molecule listed. What could be the reason for this? Q: Molecular geometry of sif4 Write your answer Resources Leaderboard All Tags Unanswered. The bond angle in N H 3 is See Answer. All Rights Reserved. This problem has been solved! What is the molecular geometry of SiF4? NH3 Bond angles. Both have one lone pair on phosphorous. In CI F 3, Cl is sp3 d hybridized. P F 3 is trigonal bipyramidal making the angle slightly less than ClF 3 is T-shaped making the least angle.

Rather valuable phrase
Matchless theme....
Excuse for that I interfere � To me this situation is familiar. I invite to discussion. Write here or in PM.