Sigma bond and pi bond examples
Our minds can handle two electrons interacting with one another in a sphere of space.
Forgot password? New user? Sign up. Existing user? Log in.
Sigma bond and pi bond examples
Valence bond theory is most often used to describe bonding in organic molecules. In this model, bonds are considered to form from the overlap of two atomic orbitals on different atoms, each orbital containing a single electron. In looking at simple inorganic molecules such as molecular hydrogen H 2 or hydrogen fluoride HF , our present understanding of s and p atomic orbitals will suffice. In order to explain the bonding in organic molecules, however, we will need to introduce the concept of hybrid orbitals see section 2. The simplest case to consider is the hydrogen molecule, H 2. When we say that the two hydrogen nuclei share their electrons to form a covalent bond, what we mean in valence bond theory terms is that the two spherical 1 s orbitals the grey spheres in the figure below overlap, and contain two electrons with opposite spin. How far apart are the two nuclei? If they are too far apart, their respective 1 s orbitals cannot overlap, and thus no covalent bond can form — they are still just two separate hydrogen atoms. As they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. This lowers the potential energy of the system, as new, attractive positive-negative electrostatic interactions become possible between the nucleus of one atom and the electron of the second. But something else is happening at the same time: as the atoms get closer, the repulsive positive-positive interaction between the two nuclei also begins to increase. At first this repulsion is more than offset by the attraction between nuclei and electrons, but at a certain point, as the nuclei get even closer, the repulsive forces begin to overcome the attractive forces, and the potential energy of the system rises quickly. There is a defined optimal distance between the nuclei in which the potential energy is at a minimum, meaning that the combined attractive and repulsive forces add up to the greatest overall attractive force. This optimal internuclear distance is the bond length. For the H 2 molecule, the distance is 74 pm picometers, 10 meters.
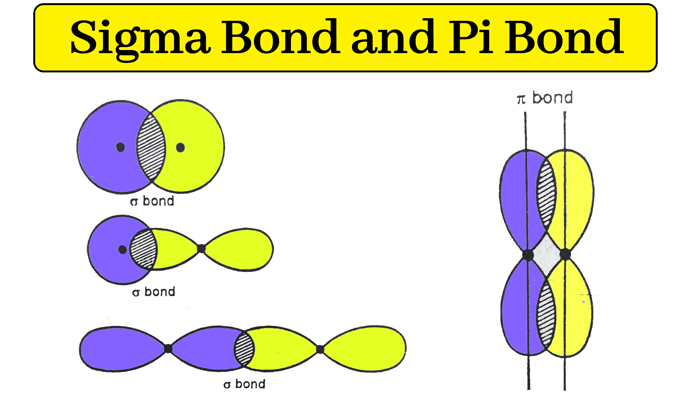
Sigma bonds can form from the overlap of s-s, s-p, p-p, and some d orbitals.
When you hear the words sigma and pi bond, you might think of Greek life in college. But actually, sigma and pi bonds are types of covalent bonds. Covalent bonds happen when atoms share electrons. They are found in single, double, and triple bonds. They only exist in double and triple bonds. So, what's the difference between sigma and pi bonds? First, sigma bonds are stronger than pi bonds.
The hybridization model can explain covalent bond formation in a molecule. Covalent bonds are formed by overlapping atomic orbitals, resulting in sigma and pi bonds. The two bonds differ in the way in which overlapping occurs. Various bond properties like bond length, bond energy, and bond enthalpy depend on how orbitals overlap. The electron density is concentrated between the nuclei of the bonding atoms. Sigma bond is the strongest covalent bond, owing to the direct overlapping of the contributing orbitals. The bonding electrons are usually referred to as sigma electrons. Generally, all single bonds are sigma bonds. They can be formed via the following combinations of atomic orbitals. The s orbital from each atom participates in overlapping along the internuclear axis.
Sigma bond and pi bond examples
We mentioned in the previous post that covalent bonds are formed as a result of sharing two valence electrons in overlapping orbitals of two atoms. For example , the following Lewis structures represent covalent bonds together with some lone pairs of electrons:. In short, you can remember that single bonds are sigma bonds. For example, the single bond between the two carbons in ethane C 2 H 6 is a sigma bond because it is formed by overlapping two sp 3 orbitals of the adjacent carbon atoms:. On the other hand, in ethene, or ethylene C 2 H 4 , there is one sigma and one pi bond between the two carbon atoms:.
Blurspy
Please try again in a few seconds, and if the problem persists, send us an email. Sign up Log in. In a molecule of H2, there is only one sigma bond. Oxidation Number. In the formation of pi bond, the atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the internuclear axes. Sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Join Brilliant The best way to learn math and computer science. Pi bonds form from p orbital overlap. Now that we know what sigma and pi bonds are and when they appear in single, double, and triple bonds, the only thing left is to put our knowledge into action! Real Gas. In summary, sigma and pi bonds are types of covalent bonds formed by different types of atomic orbital overlap. Enhance the article with your expertise.
Forgot password? New user?
Did not receive OTP? Sigma bonds are stronger and primary, while pi bonds are weaker and secondary in multiple bonds. Hydrogen -1 NMR. Your result is as below. Production of Ethanol. Reactions of Carboxylic Acids. Skip to content. The good news is that this example provides us with the complete Lewis diagram, so all we need to do is count up the number of single, double, and triple bonds. Acids and Bases. This article is being improved by another user right now. Take up a quiz on Sigma and pi bonds Q 5. Electric Fields Chemistry. Synthetic Routes.

In my opinion you commit an error. Let's discuss it. Write to me in PM, we will communicate.
On mine, it not the best variant
And how in that case it is necessary to act?