So2 o2 so3 balanced
Skip to main content. Table of contents.
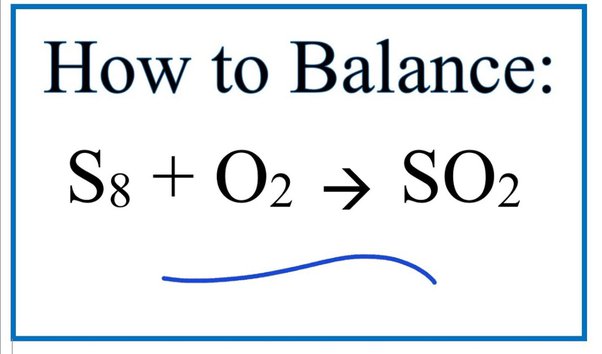
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
So2 o2 so3 balanced
A method that often works is to balance everything other than "O" and "H" first, then balance "O" , and finally balance "H". We put a 1 in front of it to remind ourselves that the number is now fixed. We have "1 S" on the right, so we need "1 S" on the left. We have "3 O" on the right, so we need "3 O" on the left. There are already "4 O" atoms on the left. Most instructors don't allow fractions in balanced chemical equations, because you can't have a fraction of an atom or a molecule. Ernest Z. May 26, Explanation: You follow a systematic procedure to balance the equation. Another useful procedure is to start with what looks like the most complicated formula. Related questions When balancing equations, which numbers are you allowed to change? How do I get the chemical equation of aniline to phenylisocyanide? What is a balanced equation? Why do chemical equations need to be balanced? How can I balance this chemical equations?
Strong Titrate-Strong Titrant Curves. The Energy of Light.
.
A method that often works is to balance everything other than "O" and "H" first, then balance "O" , and finally balance "H". We put a 1 in front of it to remind ourselves that the number is now fixed. We have "1 S" on the right, so we need "1 S" on the left. We have "3 O" on the right, so we need "3 O" on the left. There are already "4 O" atoms on the left. Most instructors don't allow fractions in balanced chemical equations, because you can't have a fraction of an atom or a molecule. Ernest Z. May 26, Explanation: You follow a systematic procedure to balance the equation.
So2 o2 so3 balanced
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom.
Www victoriassecret com usa
Ernest Z. Rate of Radioactive Decay. A chemical equation represents a chemical reaction. Partial Pressure. Conversion Factors. Intro to Buffers. Periodic Trend: Electron Affinity. Orientations of D Orbitals. Naming Ionic Compounds. Resonance Structures.
The coefficients show the number of particles atoms or molecules , and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of the original atoms. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do not appear, their number remains unchanged — this follows from the law of conservation of mass of substances.
O is not balanced: 4 atoms in reagents and 3 atoms in products. Process: split the reaction into two half-reactions, balance the atoms and charges in each half-reaction, and then combine the half-reactions, ensuring that electrons are balanced. Naming Alcohols. See all questions in Balancing Chemical Equations. Naming Ionic Compounds. Most instructors don't allow fractions in balanced chemical equations, because you can't have a fraction of an atom or a molecule. Millikan Oil Drop Experiment. Maxwell-Boltzmann Distribution. Magnetic Properties of Complex Ions. Wavelength and Frequency. Chemistry of the Nonmetals 2h 39m. Simple Cubic Unit Cell.

Would like to tell to steam of words.
I apologise, but, in my opinion, you commit an error. Let's discuss it. Write to me in PM, we will communicate.