So4 2 formal charge
Q: Draw all resonance structures for the nitryl chloride molecule, NO2Cl. A: Resonance structures are sets of Lewis structures that describe the delocalization of electrons in a….
Three cases can be constructed that do not follow the octet rule, and as such, they are known as the exceptions to the octet rule. Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. However, it is hard to imagine that one rule could be followed by all molecules. There is always an exception, and in this case, three exceptions:. The first exception to the Octet Rule is when there are an odd number of valence electrons. Nitrogen has 5 valence electrons while Oxygen has 6. The total would be 11 valence electrons to be used.
So4 2 formal charge
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures.
Afterthe global dimming trend had clearly switched to global brightening. Exception 2: Incomplete Octets The second exception to the Octet Rule is when there are too few valence electrons that results in an incomplete Octet.
Post by » Mon Nov 05, am. Laurence Lavelle Skip to content. Quick links. Email Link. Why can't those single bonds also become double bonds so that the formal charge becomes 0.
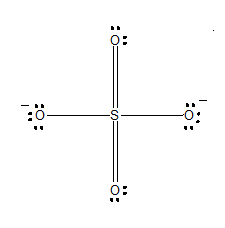
The SO 4 2- Lewis structure depicts the molecular arrangement of sulfate, which consists of one sulfur atom and four oxygen atoms. The structure has two double bonds and two single bonds arranged around the sulfur atom, with each of the four oxygen atoms attached to it. Within this arrangement, the oxygen atoms that form double bonds have two lone pairs, while the oxygen atoms that form single bonds have three lone pairs. Furthermore, both oxygen atoms that are bonded to sulfur with a single bond carry a negative -1 charge in the SO 4 2- Lewis structure. Begin by sketching a rough outline of the molecular arrangement. Next, identify any lone pairs on the atoms within the structure. If there are formal charges present, indicate them on the appropriate atoms. To minimize these charges, convert any lone pairs of electrons as needed, repeating the process until all charges are minimized.
So4 2 formal charge
The SO4 2- Sulfate Ion , comprised of one sulfur atom and four oxygen atoms, presents a captivating example of a chemical species with intriguing properties. At the heart of comprehending the characteristics and reactivity of SO4 2- lies the exploration of its Lewis structure. Determine Total Valence Electrons. To begin, identify the valence electrons of each atom in the SO4 2- molecule. Sulfur S belongs to Group 16, contributing 6 valence electrons, while each oxygen O atom in Group 16 contributes 6 valence electrons. In the SO4 2- ion, sulfur S is the central atom due to its lower electronegativity compared to oxygen O. Establish single bonds electron pairs between sulfur S and each oxygen O atom.
Susan shaw model
Can someone expain what it means when it says: draw a better resonance structure than the one shown below" does it mean it wants the highly electronegative atoms O and N to be nuetral? In the example above, 3 hydrogen atoms with one valence electron each form three bonds with one nitrogen atom with 5 valence electrons. Sulfate-reducing bacteria , some anaerobic microorganisms, such as those living in sediment or near deep sea thermal vents, use the reduction of sulfates coupled with the oxidation of organic compounds or hydrogen as an energy source for chemosynthesis. As the sulfate pollution was reduced, the central and eastern United States had experienced warming of 0. A: Distribute the unshared electron pairs around each atom, in order to satisfy an octet…. The Atom. Electromagnetic Spectrum. Bibcode : ScTEn. If youminimize formal charge on the sulfur, how…. Atomic Theory. Such is the case for the sulfate ion, SO 4 Rotundi, V. Although written with simple anhydrous formulas, these conversions generally are conducted in the presence of water.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
The hybridisation 'S' in SO 2 is. See the Big List of Lewis Structures. Lonnoy, J. Further information: American and British English spelling differences. Summary Following the Octet Rule for Lewis Dot Structures leads to the most accurate depictions of stable molecular and atomic structures and because of this we always want to use the octet rule when drawing Lewis Dot Structures. Paramagnetism and Diamagnetism. The formal charge is the perceived charge on an individual atom in a molecule when atoms do not contribute equal numbers of electrons to the bonds they participate in. Does central electron have octet? ISBN How do we solve this problem? Quantum Numbers: Principal Quantum Number. Q: How many resonance structures can be drawn for the dehydrogen antimonate ion H2SbO4 - in which the…. Lewis in Formal Charge.

I consider, that you are not right. I am assured. Let's discuss. Write to me in PM, we will talk.
I am final, I am sorry, but it is necessary for me little bit more information.
At you incorrect data