So4 lewis dot structure
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter.
Lewis structures are another way to represent molecules. Lewis Structures were introduced by Gilbert N. Lewis in Lewis suggested the use of lines between atoms to indicate bonds, and pairs of dots around atoms to indicate lone or non-bonding pairs of electrons. In the example above, 3 hydrogen atoms with one valence electron each form three bonds with one nitrogen atom with 5 valence electrons. By forming three bonds, nitrogen gains 3 electrons to make a total of 8 surrounding it.
So4 lewis dot structure
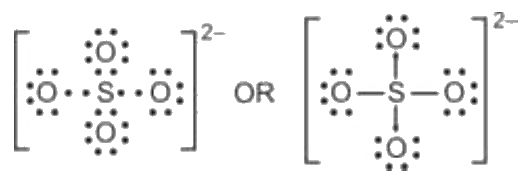
The sulfate ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4 Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid, and many are prepared from that acid. The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. First, determine the valence electron available for drawing the Lewis structure of SO 4 2- because the Lewis diagram represents valence electrons around atoms. Sulfur and oxygen atoms are located in the VIA group in the periodic table. So, oxygen and sulfur atoms have six electrons in their valence shell. Total electron pairs are determined by dividing the number of total valence electrons by two.
Boron Family Reactions. Combustion Apparatus.
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule.
It is usually easier to figure out a problem if you can draw a picture, either mental or real, of what is happening. This is often done in physics and mathematics, and it is especially helpful when looking at the bonding, structure, physical properties, and reactivity of compounds. The most common picture, or model, of elements and compounds used is the Lewis Dot Structure. These pictures show you the type s of atom s involved, their position in the molecule, and where their valence electrons are situated. Dash each dash represents two electrons that are shared between two atoms as a covalent bond. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet.
So4 lewis dot structure
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table.
Koumi-jima shuu 7 de umeru mesu tachi
Heating and Cooling Curves. The symmetry is the same as that of methane. Naming Alkenes. Oxides, Peroxides, and Superoxides. Integrated Rate Law. Drawing correct Lewis structures takes practice but the process can be simplified by following a series of steps:. Writing Formulas of Coordination Compounds. Orientations of D Orbitals. Chemical Equilibrium 0. Millikan Oil Drop Experiment.
The Sulfur atom S is at the center and it is surrounded by 4 Oxygen atoms O.
Density of Non-Geometric Objects. Partial Pressure. Arrhenius Equation. Solutions: Solubility and Intermolecular Forces. Coordination Complexes. Vapor Pressure Lowering Raoult's Law. Le Chatelier's Principle. Related articles Related Qustion. Therefore, From the octet rule, we can draw the Lewis dot structures. Lewis dot structure of SO 4 2 - :. Intensive vs. Subatomic Particles. Rutherford Gold Foil Experiment.

Very useful topic
And where logic?
Yes, really. It was and with me. We can communicate on this theme. Here or in PM.