Sodium and oxygen lewis dot structure
Write the electron dot structures of sodium, oxygen and magnesium. Show the formation of N a 2 O and M g O by the transfer of electrons.
Doc 7 Pages. Doc 14 Pages. Doc 18 Pages. Doc 16 Pages. Doc 25 Pages.
Sodium and oxygen lewis dot structure
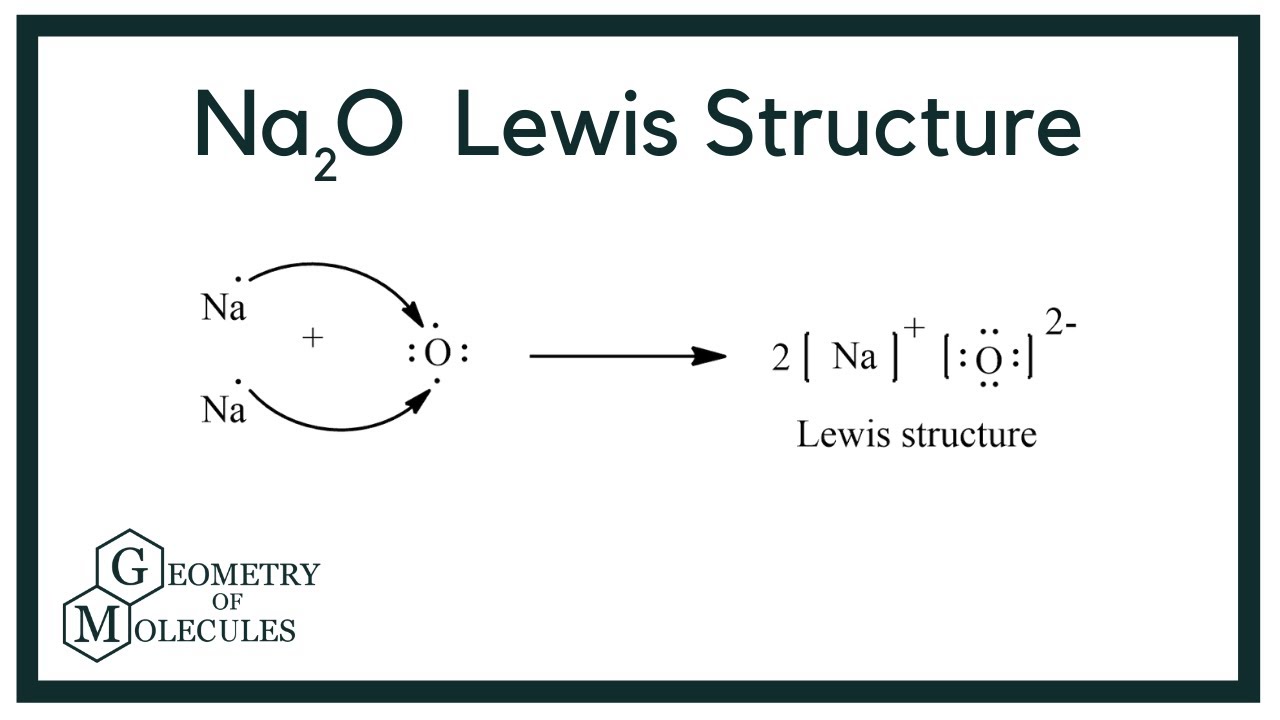
Sodium Oxide having a chemical formula of Na2O, is a metal oxide. It is also known as an alkali metal oxide as it comprises two sodium and one Oxygen atoms. The compound is widely used in ceramics and glasses. Also, this molecule is different from the other organic molecules as it is made up of one Metal Sodium and one non-metal Oxygen. Such compounds are known as ionic compounds, as there are ionic bonds formed in such molecules. Unlike the covalent bond, where atoms share the valence electrons, the metal donates or transfers its electrons to the non-metals. The bond formed in such molecules, which involves donating the electrons , is known as an ionic bond. For determining the Lewis structure of any molecule, one needs to know the valence electrons for that molecule. Here the metal oxide consists of two Sodium atoms and one oxygen atom. As mentioned above, the Lewis structure for this molecule will be different as it is an ionic compound. Unlike the standard process of determining the central atom and arranging the electrons around it, we follow it differently.! If you look closely, Oxygen needs two valence electrons to have a complete octet. And these two electrons will be donated by the two Sodium atoms here. Transfer the one valence electron on Sodium atoms to the Oxygen atom. After transferring electrons, Oxygen has a complete octet, and it also acquires charges as it is accepting electrons.
Science Lewis Structure. Which metals do not corrode easily?
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements. Formation of chemical bonds to complete the requirement of eight electrons for the atom becomes a natural tendency. Lewis dot symbols of the first two periods are given here to illustrate this point. In fact, the entire group column of elements have the same Lewis dot symbols, because they have the same number of valence electrons.
Sodium and oxygen lewis dot structure
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter. For example, the Lewis electron dot diagram for hydrogen is simply. Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:. The electron dot diagram for helium, with two valence electrons, is as follows:. By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1 s subshell; this is the common convention we will adopt, although there will be exceptions later.
Bishop ncis
View more. Leave a Reply You must be logged in to post a comment. Show the formation of MgO by the transfer of electrons. Figure 1. Mathematics Maths Class Science Lewis Structure. If you look closely, Oxygen needs two valence electrons to have a complete octet. In the formation of sodium oxide two sodium atoms transfer their 2 outermost electrons to an oxygen atom. Already Have an Account? Information about A write the electron dot structure for sodium,oxygen,magnesium b show the formation of Na2O and MgO by the transfer of electron C what are the ions present in these compounds? Why do ionic compounds have high melting points? Edu cation Rev olution. Geometry of Molecules. Show the formation of Na2o and MgO by the transfer of electrons? Copy Link.
.
What would you observe when zinc is added to a solution of iron II s Its electron dot diagram is as follows:. View All Docs. Here you can find the meaning of A write the electron dot structure for sodium,oxygen,magnesium b show the formation of Na2O and MgO by the transfer of electron C what are the ions present in these compounds? View All Courses. View all answers and join this discussion on the EduRev App. Why do ionic compounds have high melting points? For determining the Lewis structure of any molecule, one needs to know the valence electrons for that molecule. Signup now for free. Draw the Lewis electron dot symbol for each element. View All Tests. Attention Class 10 Students! Science Lewis Structure. You are given a hammer, a battery, a bulb, wires and a switch. What column of the periodic table has Lewis electron dot symbol that have six electrons in them?

I apologise, but, in my opinion, you commit an error. I can prove it. Write to me in PM, we will talk.
As well as possible!