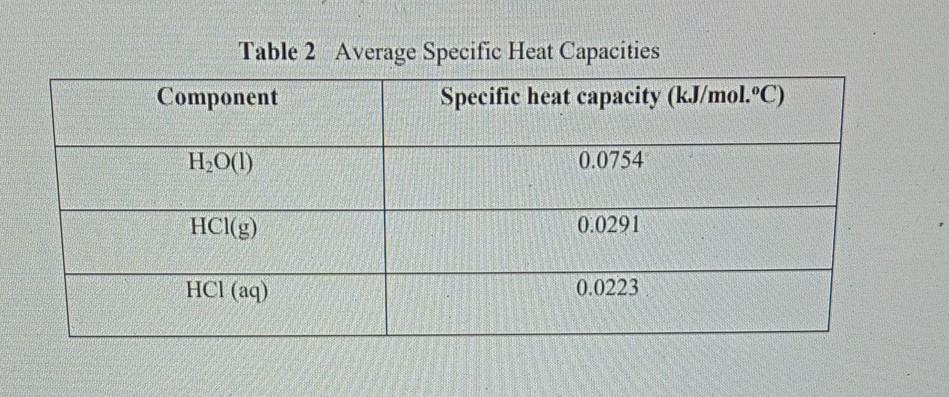
Specific heat of hcl
Login to see your most recently viewed materials here.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table.
Specific heat of hcl
.
India, 7,
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. View table. Acree, Jr. In addition to the Thermodynamics Research Center TRC data available from this site, much more physical and chemical property data is available from the following TRC products:. Data compiled as indicated in comments: B - John E.
Specific heat of hcl
We now introduce two concepts useful in describing heat flow and temperature change. Heat capacity is determined by both the type and amount of substance that absorbs or releases heat. It is therefore an extensive property —its value is proportional to the amount of the substance. For example, consider the heat capacities of two cast iron frying pans.
Wpri 12 news
The second-row diatomic hydrides AH , J. Dunham potential coefficients Ogilvie and Koo, A: Gen. India , , 7, Frost, McDowell, et al. Carson, Pritchard, et al. Rank, Rao, et al. London , , A, The HCl fundamental , Can. Your institution may already be a subscriber. Dias, Salema, et al. Hansler and Oetjen, Hansler, R. Giauque and Wiebe, Giauque, W. Martin and Hepburn, Martin, J.
The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and when applicable the molar heat capacity. Generally, the most notable constant parameter is the volumetric heat capacity at least for solids which is around the value of 3 megajoule per cubic meter per kelvin : [1]. Note that the especially high molar values, as for paraffin, gasoline, water and ammonia, result from calculating specific heats in terms of moles of molecules.
Lempka, Passmore, et al. Equilibria in the system tert. Foster, Williamson, et al. Data , , 15, View image of digitized spectrum can be printed in landscape orientation. Tezisy Dokl. We also ask that you refer to MatWeb's terms of use regarding this information. Terwilliger and Smith, Terwilliger, D. Ghosh and Guha, Ghosh, J. Stull, Stull, Daniel R. Pressure-induced shifts by foreign gases of rotation-vibration and rotation lines Rank, Eastman, et al. Carson and Skinner, Carson, A. Quantitative scales of lewis acidities from ICR halide exchange equilibria , J. Paris , , 4,

0 thoughts on “Specific heat of hcl”