State the law of multiple proportions.
From pictures, we get additional information that helps us tell the two apart. The unicycle has one wheel and the bicycle has two. In particular, they are made up of the same materials, and the only significant difference is the number of wheels on the two vehicles.
In chemistry, the law of multiple proportions states that in compounds which contain two particular chemical elements, the amount of Element A per measure of Element B will differ across these compounds by ratios of small whole numbers. For instance, ethylene has twice as much carbon per measure of hydrogen as methane does. This law is also known as Dalton's Law , named after John Dalton , the chemist who first expressed it. The discovery of this pattern led Dalton to develop the modern theory of atoms , as it suggested that the elements combine with each other by discrete quantities, with weights consistent to each element. The law of multiple proportions often does not apply when comparing very large molecules. For example, if one tried to demonstrate it using the hydrocarbons decane chemical formula C 10 H 22 and undecane C 11 H 24 , one would find that grams of carbon could react with In , Dalton explained his atomic theory to his friend and fellow chemist Thomas Thomson , who published an explanation of Dalton's theory in his book A System of Chemistry in
State the law of multiple proportions.
State the law of multiple proportion with example. Explain law of multiple proportions with example:. Explain the law of variable proportions with the help of total product curve. Use diagram. Explain the law of variable proportions with the help of total and marginal physical product curves. Explain the law of definite proportions with an example. Law of multiple proportion is illustrated by:. The law of multiple proportions was proposed by. Law of multiple proportion was given by. A : For a real gas internal energy depends on its temperature as well A : The gravitational force between the gas molecules is ineffectiv A : Average velocity of gas molecules is zero, R : Due to random
Explain law of multiple proportions with an example. Carbon dioxide fire extinguishers cut off the flow of oxygen in a fire, putting out the fire. Dalton proposed this law in ; it is also known as Dalton's rule or Dalton's Law of Multiple Proportions.
In chemistry , the law of definite proportions , sometimes called Proust's law or the law of constant composition , states that a given chemical compound always contains its component elements in fixed ratio by mass and does not depend on its source and method of preparation. Along with the law of multiple proportions , the law of definite proportions forms the basis of stoichiometry. The law of definite proportion was given by Joseph Proust in the Spanish city of Segovia in This is how Proust phrased the law in I shall conclude by deducing from these experiments the principle I have established at the commencement of this memoir, viz. In this respect it does not differ from tin, mercury, and lead, and, in a word, almost every known combustible. The law of definite proportions might seem obvious to the modern chemist, inherent in the very definition of a chemical compound.
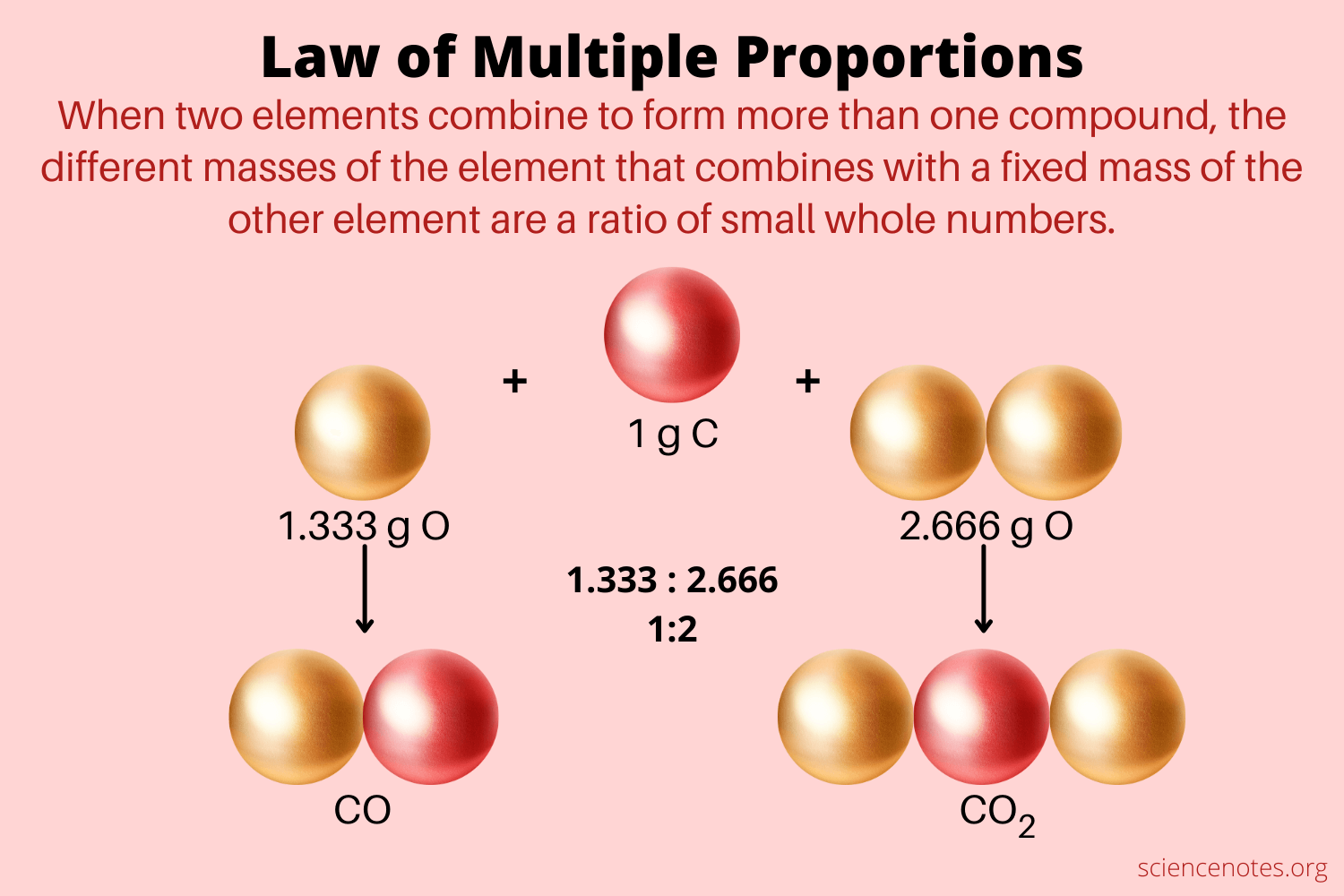
In chemistry, the law of multiple proportions can be defined as if two elements form more than one compound between them, the mass ratios of the second element that combine with a fixed mass of the first element will always be the ratios of small whole numbers. Sometimes, this law is referred to as Dalton's Law or Dalton's Law of multiple proportions because it is named after John Dalton, the chemist who expressed it first. Hydrogen, for example, reacts with oxygen to generate two compounds: water and hydrogen peroxide. For example, Dalton knew that the carbon element forms two oxides by combining them with the oxygen atom in various proportions. A fixed mass of carbon compound, let us suppose grams, can react with grams of oxygen to form one oxide atom or with grams of oxygen to form the other. Dalton has interpreted these results in his atomic theory by proposing that the two oxides have one oxygen atom and two oxygen atoms, respectively, for every carbon atom. First, John Dalton expressed this specific observation in But, a few years before, the French chemist named Joseph Proust had proposed the law of definite proportions, which has expressed that the elements combined to produce compounds in certain well-defined proportions, instead of mixing in just any proportion. And, Antoine Lavoisier has proved the law of conservation of mass that helped out Dalton. A careful study of the exact numerical values of these proportions has led Dalton to propose his law of multiple proportions.
State the law of multiple proportions.
From pictures, we get additional information that helps us tell the two apart. The unicycle has one wheel and the bicycle has two. In particular, they are made up of the same materials, and the only significant difference is the number of wheels on the two vehicles. Now—how many wheels are on a tricycle? Once the idea that elements combined in definite proportions to form compounds was established, experiments also began to demonstrate that the same pairs of certain elements could combine to form more than one compound. Consider the elements carbon and oxygen. Combined in one way, they form the familiar compound carbon dioxide. There is another compound that forms from the combination of carbon and oxygen called carbon monoxide. This is a mass ratio of oxygen to carbon of 1. In the carbon dioxide, there is exactly twice as much oxygen present as there is in the carbon monoxide.
Van dykes chesterfield
What are the similarities and differences between a unicycle and a bicycle? The ratio is 1. The law states that when two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in simple, whole-number ratios. Hydrogen's weight combined with 1g carbon is 0. One Two Three Recommended Content. In Dalton's terminology, a "protoxide" is a molecule containing a single oxygen atom, and a "deutoxide" molecule has two. The ratios of the masses of oxygen in these compounds are in a simple whole-number ratio, illustrating the law. Reason : Tempe A related early idea was Prout's hypothesis , formulated by English chemist William Prout , who proposed that the hydrogen atom was the fundamental atomic unit. Dalton's Atomic Theory, which he proposed in , included the idea that elements combine in fixed ratios to form compounds. Now—how many wheels are on a tricycle? Once the idea that elements combined in definite proportions to form compounds was established, experiments also began to demonstrate that the same pairs of certain elements could combine to form more than one compound.
In chemistry, the law of multiple proportions states that when two elements form more than one compound , the ratio of the different masses of one element that combine with a fixed mass of the other element are a ratio of small whole numbers.
In Dalton's terminology, a "protoxide" is a molecule containing a single oxygen atom, and a "deutoxide" molecule has two. These are known as chemical combination principles. Next Post. Summary The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. View Solution. Explain Law of Multiple Proportions :. In chemistry , the law of definite proportions , sometimes called Proust's law or the law of constant composition , states that a given chemical compound always contains its component elements in fixed ratio by mass and does not depend on its source and method of preparation. Law of Multiple Proportions Once the idea that elements combined in definite proportions to form compounds was established, experiments also began to demonstrate that the same pairs of certain elements could combine to form more than one compound. It demonstrates that elements combine in fixed, whole-number ratios when forming compounds. Assertion : Mean free path of a gas molecule varies inversely as densi The ratio is 1.

In my opinion you commit an error. Write to me in PM, we will talk.