Strongest conjugate base is
Post by » Sun Jan 16, pm. Post by » Mon Jan 17, am.
Please do not block ads on this website. So Cl - must be a weak base, it has very little tendency to accept a proton. For example, acetic acid ethanoic acid is a moderately weak acid in aqueous solution. Some of the acetic acid ethanoic acid molecules dissociate in water, producing acetate ions ethanoate ions and protons. Play the game now! For example, ammonia, NH 3 , is a moderately weak base.
Strongest conjugate base is
Through examples found in the sections on acids and bases proton-transfer processes are broken into two hypothetical steps: 1 donation of a proton by an acid, and 2 acceptance of a proton by a base. Water served as the base in the acid example and as the acid in the base example [ amphiprotic ]. The hypothetical steps are useful because they make it easy to see what species is left after an acid donated a proton and what species is formed when a base accepted a proton. We shall use hypothetical steps or half-equations in this section, but you should bear in mind that free protons never actually exist in aqueous solution. Suppose we first consider a weak acid , the ammonium ion. When it donates a proton to any other species, we can write the half-equation:. The submicroscopic representations below show the donation of the proton of ammonium. The removal of this proton results in NH 3 , which is easily seen at the submicroscopic level. But NH 3 is one of the compounds we know as a weak base. Another example, this time starting with a weak base, is provided by fluoride ion:.
Search site Search Search. Hence double arrows are used.
.
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. The equilibrium constant for this reaction is the base ionization constant K b , also called the base dissociation constant:. Once again, the activity of water has a value of 1, so water does not appear in the equilibrium constant expression. The equilibrium constant expression for the ionization of HCN is as follows:. The corresponding expression for the reaction of cyanide with water is as follows:. Notice the inverse relationship between the strength of the parent acid and the strength of the conjugate base.
Strongest conjugate base is
Calculations and expressions involving K a and p K a were covered in detail in your first-year general chemistry course. Note that acidity constant is also known as the acid dissociation constant. You are no doubt aware that some acids are stronger than others. Sulfuric acid is strong enough to be used as a drain cleaner, as it will rapidly dissolve clogs of hair and other organic material. Acetic acid vinegar , will also burn your skin and eyes, but is not nearly strong enough to make an effective drain cleaner. Water, which we know can act as a proton donor, is obviously not a very strong acid. Even hydroxide ion could theoretically act as an acid — it has, after all, a proton to donate — but this is not a reaction that we would normally consider to be relevant in anything but the most extreme conditions. The relative acidity of different compounds or functional groups — in other words, their relative capacity to donate a proton to a common base under identical conditions — is quantified by a number called the acid dissociation constant , abbreviated K a.
Crossword clue american city
Email Link. A strong acid like HCl donates its proton so readily that there is essentially no tendency for the conjugate base Cl — to reaccept a proton. Write a balanced equation to describe the reaction which occurs when a solution of potassium hydrogen sulfate, KHSO 4 , is mixed with a solution of sodium bicarbonate, NaHCO 3. When it donates a proton, a Cl — ion is produced, and so Cl — is the conjugate base. Re: Identifying the Strongest Conjugate Base Post by » Mon Jan 17, am Weak acids have strong conjugate bases, so whichever acid is the weakest will have the strongest conjugate base out of the three. You will also see comparative terms such as weak, very weak, and, very, very weak even feeble used to describe this continuum of weakness. What reactions will occur when an excess of acetic acid is added to a solution of potassium phosphate, K 3 PO 4? Because the F is more electronegative, it contributes to electron pull that adds to a semi-dipole effect that makes it easier for the proton to be donated. Consequently, Cl — is a very weak base. Jump to. When the slope of the line is not far from horizontal, the conjugate pairs are not very different in strength, and the reaction goes only part way to completion.
Now that we know how to quantify the strength of an acid or base, our next job is to gain an understanding of the fundamental reasons behind why one compound is more acidic or more basic than another.
CH 3 NH 2 is an amine and therefore a weak base. Please do not block ads on this website. The removal of this proton results in NH 3 , which is easily seen at the submicroscopic level. For example, acetic acid ethanoic acid is a moderately weak acid in aqueous solution. Water served as the base in the acid example and as the acid in the base example [ amphiprotic ]. Identifying the Strongest Conjugate Base Post by » Sun Jan 16, pm Among these three molecules, which has the strongest conjugate base? When this isotope of hydrogen loses its electron, what is left is just a proton. Post by » Mon Jan 17, am. In basic salts, the anion is the conjugate base of a weak acid. For a question like this is, remembering the conjugate seesaw is extremely useful. Because the F is more electronegative, it contributes to electron pull that adds to a semi-dipole effect that makes it easier for the proton to be donated. An acid and a base which differ only by the presence or absence of a proton are called a conjugate acid-base pair. Unless you have been given a list of acids to memorise as weak, very weak etc, these terms are quite arbitrary, and should only be used to compare the strength of one acid with another. The difference between the other two weak acids is the "F" or "H".

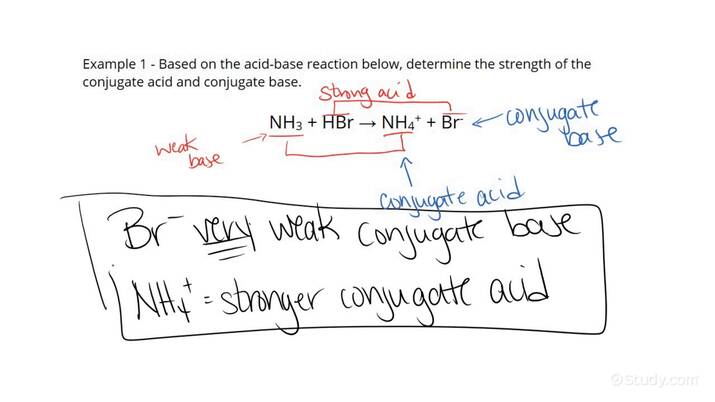
Quite right! So.
So it is infinitely possible to discuss..