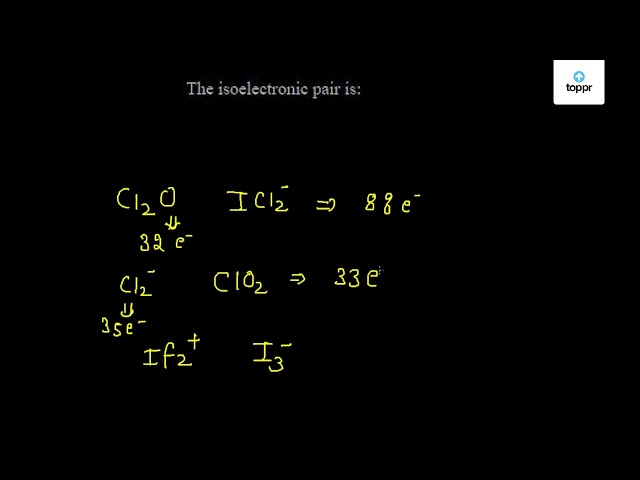
The isoelectronic pair is
Isoelectronic refers to two ions or molecules having the same electronic structure and the same number of valence electrons. Last updated on Dec 20, Candidates must go through the NDA1 previous year's papers. Attempting the NDA1 mock tests is also essential.
The isoelectronic pairs is :. The isoelectronic pair of ions is -. The isoelectronic pair of 32 electrons is. Which of the following pair s represent s the isoelectronic species? The group having isoelectronic species is Which of the following is isoelectronic pair? The group having isoelectronic species is.
The isoelectronic pair is
Isoelectronic means "same electronic structure". For atoms, we can say the "same number of valence electrons". We can assume that if an atom and an ion or two ions have the same number of electrons, they will be arranged in the same shells and subshells. Chemistry Matter Net Charge. Dec 15, Explanation: Isoelectronic means "same electronic structure". The calcium and chloride ions both have 18 electrons. Related questions What determines if an atom is electrically charged or electrically neutral? What is the law of conservation of electric charge? Can a polarized object have a net charge of zero? What is the SI unit of electric charge? What is the elementary charge? What does it mean that the electric charge is quantized?
Overall, isoelectronic species are having the same number of electrons only. JEE Main Syllabus.
Answer: Ions or atoms with a similar amount of electrons are referred to as isoelectronic species. The number of electrons within the isoelectronic species is identical, but certain elements are not identical. Isoelectronic species refers to atoms ions that have a similar quantity of electrons. As a result, the isoelectronic species with F — must have 10 electrons. Isoelectronic consists of 2 ions, atoms, or molecules with identical electronic structures and valence electron numbers.
The observation that isoelectronic species are usually isostructural, first made by Penny and Southerland in , known as the isoelectronic principle Geoff. Table 1 shows an example of isostructural isoelectronic species periodic trends. All of these molecules are octahedral and isoelectronic within their periods. Search site Search Search. Go back to previous article. Sign in. Isoelectronic Species Periodic Trends The observation that isoelectronic species are usually isostructural, first made by Penny and Southerland in , known as the isoelectronic principle Geoff. Isoelectronic arrays--an atom is replaced with another which alters the charge and continues the isoelectronic relationship.
The isoelectronic pair is
Atomism, because it was dismissed by Aristotle, enjoyed a long sleep in scientific discourse until it was reconsidered by Galileo, Decartes, and Gassendi in the s. Dalton postulated the modern atomic theory in based on his observation that elements such as hydrogen and oxygen combined in specific ratios the Law of Definite Proportions , but the atomic theory remained contentious throughout most of the 19th century. Thompson, Rutherford, Bohr, and others around the turn of the 20th century established that matter was indeed composed of atoms that contained heavy nuclei and light electrons, and that atoms could exist in excited states that could be interpreted as excitations of their electrons to different energy levels. However the atomic theory did not provide a ready explanation for the bonded states of atoms in molecules.
Facialporno
Free study material. JEE Advanced Cutoff. In which of the following there exists a p pi-p pi bonding. Teaching Exams. ICAR Technician. Rajasthan PTET. RRB Junior Translator. Both species have the same number of electrons, so it is an isoelectronic pair. NHB Assistant Manager. West Bengal Judicial Service. JEE Main Coaching. Gujarat TAT. Maharashtra Nagar Parishad Engineering Services.
An ion is formed when either one or more electrons are removed from a neutral atom to form a positive ion cation or when additional electrons attach themselves to neutral atoms to form a negative one anion. The designations cation or anion come from the early experiments with electricity which found that positively charged particles were attracted to the negative pole of a battery, the cathode, while negatively charged ones were attracted to the positive pole, the anode. Ionic compounds consist of regular repeating arrays of alternating positively charged cations and negatively charges anions.
Rajasthan CET. Candidates must go through the NDA1 previous year's papers. Because features of isoelectronic species are supposed to be consistent and predictable, recognizing a chemical as isoelectronic with one already defined provides indications of likely properties and reactions. TN TET. Note: The importance of the concept lies in identifying related species, as pairs or series. Water has high heat of vaporisation due to? Gujarat Police. Indian Navy Tradesman. EMRS Librarian. Gujarat TET Exam. RBI Grade B. CISF Driver. How many images will be formed if angle between mirrors is 30 degree?

Willingly I accept. An interesting theme, I will take part.